Please note: a valid prescription is required for all prescription medication.
Soliqua Solostar Pens Uses, Dosing Basics, and Safety
Offer extended: Use code SAVE10 for 10% OFF all RX meds now through March 10, 2026. Ozempic from Canada and Mounjaro Vial not included. Coupon code cannot be combined with other offers. For products with “Bulk Savings”, the discount will be applied to the regular price for 1 unit. Maximum allowable quantity equal to a 90 day supply per single order.
$290.99
You save


Soliqua SoloStar is a prescription prefilled injection pen that combines two diabetes medicines in one daily shot. People may search for buy Soliqua Solostar Pens after being prescribed this option for type 2 diabetes management. This page explains what it is, how it works, dosing basics, safety topics, storage, and practical handling points to review with a clinician.
What Soliqua Solostar Pens Is and How It Works
Soliqua SoloStar contains insulin glargine (a long-acting or “basal” insulin) and lixisenatide, a GLP-1 receptor agonist (an incretin-mimetic that helps regulate meal-related glucose). Together, these components target both fasting glucose and post-meal glucose rises, supporting glycemic control (blood sugar control) for appropriate patients. CanadianInsulin works as a prescription referral service and may confirm details with your prescriber.
Some patients explore Ships from Canada to US when comparing pharmacy fulfillment options for this prescription medicine. The insulin glargine part helps lower glucose between meals and overnight by improving glucose uptake and reducing liver glucose output. The lixisenatide part can increase glucose-dependent insulin release, reduce glucagon after meals, and slow gastric emptying (how quickly food leaves the stomach), which can affect after-meal readings. Dispensing is handled by licensed partner pharmacies where permitted.
Because this is a fixed-dose combination, the pen delivers both ingredients in a set ratio each time the dose is dialed. That can simplify routines for people who otherwise might use a basal insulin plus a separate GLP-1 injection. For background on how diabetes medicines fit together, you can browse hubs like Diabetes Medications and Glp 1 Agonists.
Who It’s For
Soliqua SoloStar is used to improve glycemic control in adults with type 2 diabetes, when prescribed as part of a broader care plan that can include nutrition, activity, and other medications. It is generally considered when a clinician wants both basal insulin coverage and GLP-1–based effects in a single daily injection. It is not used for type 1 diabetes and is not intended to treat diabetic ketoacidosis (a medical emergency related to severe insulin deficiency).
Clinicians usually review medical history and current therapies before selecting a fixed-ratio combination. Key considerations may include prior use of insulin or GLP-1 medicines, current A1C and fasting glucose patterns, kidney function, and gastrointestinal conditions. People with a history of pancreatitis, severe stomach or bowel problems (including significant gastroparesis), or frequent hypoglycemia may need closer review. For condition context and related resources, see Type 2 Diabetes and the Type 2 Diabetes article hub.
Dosage and Usage
Soliqua SoloStar is injected under the skin (subcutaneous injection) once daily, typically timed before the first meal of the day, following the product label and prescriber instructions. This medicine is not taken by mouth and is not injected into a vein or muscle. Because the pen contains both a basal insulin and a GLP-1 receptor agonist, the dose is individualized by the prescriber based on prior treatment and glucose monitoring results.
In online searches, the phrase buy Soliqua Solostar Pens often appears alongside questions about how to use the pen safely. General pen-use steps include attaching a new needle each time, priming as directed in the instructions for use, injecting into recommended sites, and rotating injection locations to reduce skin changes such as lipohypertrophy (fatty lumps under the skin). For practical technique refreshers, review How To Use Insulin Pen, Insulin Pen Needles, and Mounjaro Injection Sites.
Quick tip: If a dose looks incomplete, document what happened and contact the prescriber for next-step guidance.
Do not share pens between people, even if the needle is changed, because blood exposure can occur. Also avoid mixing the pen’s contents with other insulins in the same syringe. If you use other diabetes medicines, a clinician may adjust the overall regimen to reduce the risk of low blood sugar, especially when sulfonylureas or additional insulin are involved.
Strengths and Forms
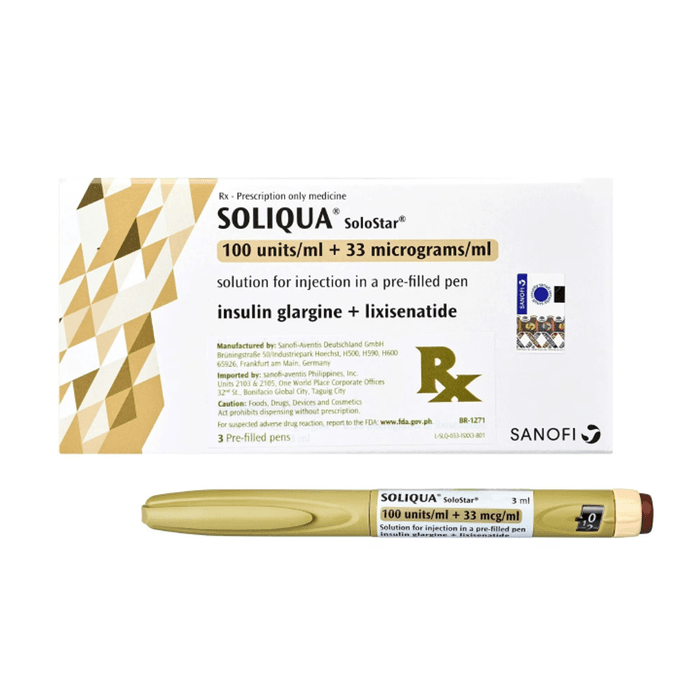
Soliqua SoloStar is supplied as a disposable prefilled pen for subcutaneous injection. It contains a fixed ratio of insulin glargine and lixisenatide, so each unit dialed delivers both medicines together. Availability can vary by jurisdiction and pharmacy channel, and packaging may be listed as a multi-pen carton (often a 5-pen presentation) depending on the source.
When patients look up buy Soliqua Solostar Pens, they often want to confirm what “100/33” means. The commonly referenced concentration is insulin glargine 100 units/mL with lixisenatide 33 mcg/mL. The pen’s dose window and maximum dialable dose are defined by the device design and the label, which matters if a prescriber is titrating toward a target fasting glucose. For a broader overview of devices used in diabetes care, see Understanding Diabetes Tech.
| Feature | What to know |
|---|---|
| Medicine class | Basal insulin plus GLP-1 receptor agonist |
| Route | Subcutaneous injection |
| Device | Disposable prefilled pen; new needle each use |
| Label specifics | Follow instructions for use and dosing plan |
Because it is a combination product, switching to or from other insulin or GLP-1 therapies should be managed by a prescriber. Keep the carton and pen labeling available, since they include device instructions, concentration information, and the in-use storage guidance.
Storage and Travel Basics
Store Soliqua SoloStar according to the product labeling to protect potency and reduce device issues. Unused pens are generally kept refrigerated and protected from light; do not freeze, and do not use a pen that has been frozen. Avoid exposing the pen to excessive heat, such as a hot car or direct sunlight near a window.
Once a pen is in use, the label specifies how long it can be kept and at what temperature range. Many insulin pens have an “in-use” period and a maximum room-temperature limit; Soliqua SoloStar’s instructions should be followed rather than relying on general insulin rules. For travel planning and temperature control concepts that also apply to many injectables, see How To Travel With Ozempic.
Why it matters: Temperature swings can reduce effectiveness even when the device looks normal.
When traveling, keep supplies together: the pen, spare pen needles, alcohol swabs if used in your routine, and a method for safe needle disposal. If you use glucose monitoring, bring extra test strips or sensors as needed. Carry medication in your hand luggage when flying, and keep the prescription label available for identification.
Side Effects and Safety
Soliqua SoloStar can cause side effects related to either component. Gastrointestinal effects such as nausea, vomiting, diarrhea, and decreased appetite may occur, especially when starting or titrating. Because it contains insulin, hypoglycemia (low blood sugar) is a key safety risk, and the risk can increase if used with other glucose-lowering medicines or with missed meals. Injection-site reactions like redness or itching can occur as well.
People searching buy Soliqua Solostar Pens also commonly look for serious warning signs that require urgent medical help. Seek care for symptoms of severe low blood sugar (confusion, seizure, loss of consciousness), signs of pancreatitis (persistent severe abdominal pain that may radiate to the back, with or without vomiting), severe allergic reaction (swelling of face or throat, trouble breathing), or significant dehydration from vomiting or diarrhea. Kidney problems can worsen in the setting of dehydration, so clinicians often emphasize hydration and monitoring when GI symptoms are significant.
Monitoring plans vary, but many patients check fasting glucose patterns during dose adjustments and track symptoms that could suggest intolerance. If you take multiple diabetes medicines, coordination matters; a helpful overview is Acceptable Combinations Of Diabetes. For general medication context, Common Diabetes Medications can help you understand how different drug classes act in the body.
Drug Interactions and Cautions
Drug interactions and precautions for Soliqua SoloStar relate to both insulin glargine and lixisenatide. Any medicine that lowers blood glucose can increase the chance of hypoglycemia when combined, including sulfonylureas and additional insulin products. Alcohol can also complicate glucose management by increasing hypoglycemia risk and impairing symptom recognition.
Lixisenatide can slow gastric emptying, which may change how quickly some oral medicines are absorbed. This may be relevant for medicines with a narrow therapeutic range (where small changes in drug levels matter) or where timing is critical. Clinicians may recommend timing adjustments for certain oral therapies based on the label and the specific medication. Also tell the prescriber about kidney disease, recurring severe stomach symptoms, or prior pancreatitis, since those factors may influence whether this combination is appropriate.
If you use other injectable therapies, avoid combining overlapping incretin-based treatments unless specifically directed by a prescriber. Bringing a complete medication list, including over-the-counter products and supplements, helps reduce interaction risks and prevents duplicate therapy. If you are unsure which medicines are in the same class, a pharmacist or prescriber can review your regimen for therapeutic overlap.
Compare With Alternatives
Soliqua SoloStar is one approach among several for type 2 diabetes management. A common alternative is using a basal insulin and a GLP-1 receptor agonist as separate prescriptions, which can allow more flexibility in adjusting each component. Another approach is using basal insulin alone, or using a GLP-1 medicine alone, depending on glucose patterns, A1C goals, tolerability, weight considerations, and contraindications.
If someone searches buy Soliqua Solostar Pens, they may also be comparing it with weekly GLP-1 options that do not contain insulin, such as Ozempic Semaglutide Pens. Some patients compare it with other weekly injectables that act on incretin pathways, such as Mounjaro Kwikpen Pre Filled Pen, though those products have different ingredients and dosing schedules. The key practical difference is that Soliqua SoloStar includes basal insulin, which can be helpful for fasting hyperglycemia but also raises hypoglycemia considerations compared with GLP-1–only therapy.
When comparing options, focus on administration frequency, device comfort, expected monitoring needs, and whether your current regimen already includes insulin. It can also help to discuss whether GI side effects are a concern, how kidney function affects choices, and what “step therapy” rules may apply through a particular health plan.
Pricing and Access
Coverage and access for Soliqua SoloStar vary across plans and regions, and this medicine requires a valid prescription. Some patients who research buy Soliqua Solostar Pens are trying to understand why one person’s out-of-pocket amount differs from another’s. Common drivers include formulary status, prior authorization requirements, deductible phase, and whether a plan prefers separate basal insulin and GLP-1 products instead of a fixed-ratio combination.
CanadianInsulin can help coordinate prescription details when confirmation from the prescriber is needed. Documentation may include the written prescription, the intended dosing range, and confirmation of current therapy to prevent duplication with other insulin or GLP-1 medicines. For planning discussions about budget impact without insurance, it may help to review general tips in Out Of Pocket Cost, then apply those concepts to your specific coverage and eligibility. Cross-border fulfillment and cash-pay options depend on eligibility and jurisdiction.
Access is also affected by clinical considerations, such as whether a patient has tolerated GLP-1 therapy before, how insulin doses are being adjusted, and whether other glucose-lowering medicines will be continued. If a plan does not cover the combination, clinicians sometimes evaluate alternatives that achieve similar goals through separate prescriptions, while keeping safety monitoring straightforward.
Authoritative Sources
For official prescribing details and medication guide language, consult the monograph on DailyMed.
For broader standards of diabetes care and monitoring, review guidance from the American Diabetes Association.
Temperature-sensitive medicines are commonly packed using prompt, express, cold-chain shipping to help maintain labeled storage conditions.
This content is for informational purposes only and is not a substitute for professional medical advice.
Express Shipping - from $25.00
Shipping with this method takes 3-5 days
Prices:
- Dry-Packed Products $25.00
- Cold-Packed Products $35.00
Standard Shipping - $15.00
Shipping with this method takes 5-10 days
Prices:
- Dry-Packed Products $15.00
- Not available for Cold-Packed products
What is Soliqua SoloStar and what does it treat?
Soliqua SoloStar is a prescription injectable medicine for adults with type 2 diabetes. It combines insulin glargine (a long-acting basal insulin) with lixisenatide (a GLP-1 receptor agonist) in a single prefilled pen. The goal is improved blood sugar control, covering both fasting glucose and post-meal rises. It is not used for type 1 diabetes and is not for treating diabetic ketoacidosis. A clinician decides whether it fits your overall plan and what other diabetes medicines should be continued or stopped.
How is Soliqua different from Ozempic or other GLP-1 medications?
Soliqua includes both a basal insulin and a GLP-1 receptor agonist, so each dose delivers two medicines together. Ozempic and several other GLP-1 medications contain only a GLP-1 ingredient and do not include insulin. Practically, that means Soliqua can be useful when fasting glucose remains high and basal insulin is needed, but it also adds insulin-related risks like hypoglycemia. Dosing schedules and titration approaches differ across products, so comparisons should be based on the official label and clinician guidance.
When should Soliqua be injected during the day?
Soliqua is typically administered once daily and is commonly timed before the first meal of the day, following the product labeling and your prescriber’s instructions. Consistent timing can make glucose patterns easier to interpret during dose adjustments. Because it is a fixed-ratio combination, it should not be mixed with other insulins in a syringe, and it should not be used as a substitute for mealtime (rapid-acting) insulin unless a clinician specifically changes your regimen. Review the instructions for use for priming and injection technique.
What side effects should be monitored while using Soliqua?
Common side effects can include nausea, vomiting, diarrhea, decreased appetite, and injection-site reactions. Because Soliqua contains insulin, low blood sugar is an important risk to monitor, especially if other glucose-lowering medicines are also used. Seek urgent care for signs of severe hypoglycemia (confusion, seizure, unconsciousness), a severe allergic reaction (swelling, breathing difficulty), or possible pancreatitis (persistent severe abdominal pain, sometimes with vomiting). If gastrointestinal symptoms are significant, clinicians may also monitor hydration and kidney function.
What should I ask my clinician or pharmacist before starting Soliqua?
Bring a complete list of your diabetes medicines and doses, including any insulin, sulfonylureas, or GLP-1 therapies, and ask whether anything should be discontinued to avoid duplication. Ask how to time the injection with meals, what glucose readings to track during titration, and what to do if a dose is missed. It is also reasonable to ask about pancreatitis history, kidney function, and significant stomach or bowel symptoms, since these can affect suitability. Finally, confirm storage rules and the in-use period for opened pens.
Can Soliqua affect other oral medications I take?
Yes, it can. The lixisenatide component may slow gastric emptying, which can change how quickly certain oral medications are absorbed. This is most important for medicines where timing is critical or where small changes in blood levels matter. Tell your clinician about all prescription and non-prescription products you take, including supplements. If there is a concern, the prescriber may recommend spacing certain tablets away from the injection or monitoring more closely when starting. Do not adjust timing on your own without guidance.
Rewards Program
Earn points on birthdays, product orders, reviews, friend referrals, and more! Enjoy your medication at unparalleled discounts while reaping rewards for every step you take with us.
You can read more about rewards here.
POINT VALUE
How to earn points
- 1Create an account and start earning.
- 2Earn points every time you shop or perform certain actions.
- 3Redeem points for exclusive discounts.
You Might Also Like
Related Articles
Ozempic Side Effects In Females: Symptoms, Risks, Next Steps
Overview Ozempic (semaglutide) is a GLP-1 receptor agonist medication used for type 2 diabetes. Some people also discuss it in the context of weight management. This article focuses on ozempic…
Zepbound Side Effects Long-Term: What to Monitor Over Time
Key Takeaways Most effects are gastrointestinal and often improve over time. You may notice symptoms soon after the first injection. Track patterns: timing, triggers, and recovery after each dose. zepbound…
GLP-1 Drugs for Weight Loss: Options, Risks, and Next Steps
Key Takeaways These medicines target appetite signals and digestion to support weight management. Approval and use vary by product, condition, and country-specific labeling. Many options are injections; one GLP-1 medicine…
SGLT2 Inhibitors Mechanism of Action in Heart Failure
Overview Heart failure is not just a “weak heart.” It is a body-wide syndrome that affects fluid balance, kidneys, and energy use. Understanding sglt2 inhibitors mechanism of action in heart…