Please note: a valid prescription is required for all prescription medication.
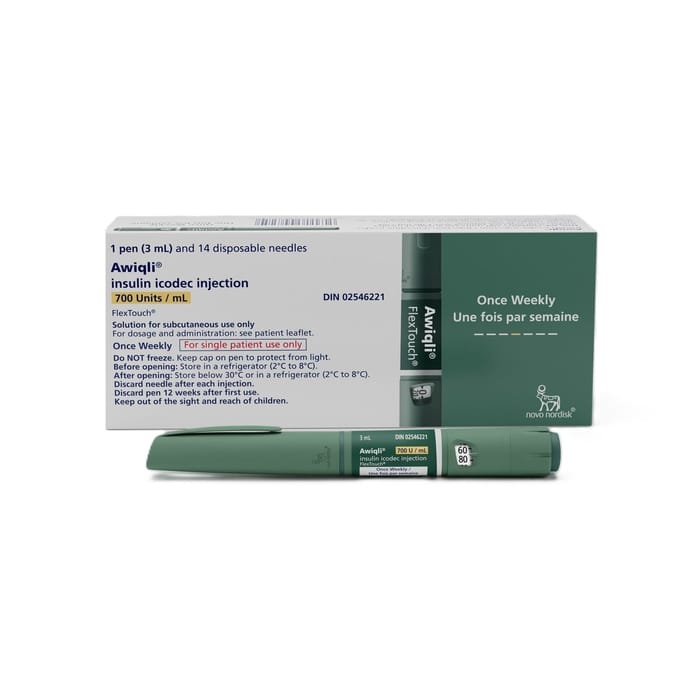
Awiqli FlexTouch Pen is a prefilled insulin pen that contains insulin icodec, a long-acting basal insulin designed for once-weekly dosing. It is used as part of an overall diabetes treatment plan to help manage background (basal) insulin needs. This page explains how it works, basic use and handling, safety considerations, and practical access topics.
What Awiqli FlexTouch Pen Is and How It Works
This medicine is a weekly insulin pen intended to provide a steady “background” insulin effect between doses. Basal insulin supports day-to-day glucose control, especially overnight and between meals, and it is different from mealtime (rapid-acting) insulin that targets post-meal rises. CanadianInsulin operates as a prescription referral service rather than a dispensing pharmacy, which helps keep the process aligned with prescriber directions.
Insulin icodec is engineered to stay active longer in the body than daily basal insulins, allowing a once-weekly insulin pen schedule in settings where it is authorized. Some patients explore Ships from Canada to US as one option when cross-border fulfilment is permitted by jurisdiction and eligibility. Because labeling and authorization can vary by country, the safest approach is to rely on the product monograph or prescribing information that matches where the prescription is written and filled.
Why it matters: Weekly dosing can change how missed doses and transitions are managed.
Who It’s For
Basal insulin therapy is commonly used for people with diabetes who need ongoing insulin support in addition to lifestyle measures and, for some, other glucose-lowering medicines. Depending on the local label, insulin icodec products may be used in adults who require basal insulin as part of their regimen. Clinicians may consider factors such as prior insulin exposure, risk of low blood sugar (hypoglycemia), and the ability to follow a consistent weekly schedule.
This treatment is not the same as rapid-acting insulin and is not intended for the urgent management of diabetic ketoacidosis (DKA). It may also be unsuitable for people with a known hypersensitivity to insulin icodec or to any inactive ingredients in the formulation. If you use an insulin pump or require very frequent short-acting corrections, a clinician will usually review whether a weekly basal insulin fits your overall plan.
For site navigation and related education, you can browse the Diabetes Condition Hub or the Diabetes Product Category to see other diabetes-related items and resources.
Dosage and Usage
Awiqli FlexTouch Pen is designed for subcutaneous injection (under the skin) on a once-weekly schedule, typically on the same day each week. The exact unit dose and any transition plan from a daily basal insulin should come from the prescriber and the official label. When switching from another basal insulin, clinicians may use a structured conversion approach and may recommend closer glucose monitoring during the changeover.
Using a prefilled pen usually involves attaching a new pen needle, priming as directed, selecting the dose on the dial, injecting into recommended sites (such as abdomen, thigh, or upper arm), and rotating sites to reduce skin problems. Injection technique matters because shallow injections, repeated use of needles, or injecting into lipohypertrophy (thickened fatty tissue) can make absorption less predictable. If a dose is missed or delayed, follow the product instructions and prescriber guidance rather than “making up” doses independently.
Quick tip: Keep a written log of injection day, site, and dose for consistency.
If you prefer to browse education by topic, the Diabetes Articles Category is a hub of related reading.
Strengths and Forms
Awiqli FlexTouch Pen is a prefilled pen device intended for single-patient use. Basal insulin pens are typically labeled in insulin units (IU) and are used with compatible single-use pen needles (often sold separately). The carton and pen label list the concentration, total volume, and the dose increments that the device can deliver.
Because available presentations can differ by region, confirm the exact strength, pack size, and needle compatibility on the package you receive and in the local prescribing information. If anything on the prescription or labeling is unclear, a pharmacist can help verify the intended product and ensure it matches the prescriber’s directions before use.
Storage and Travel Basics
Insulin products are sensitive to temperature and light exposure. In general, unopened pens are stored refrigerated according to the label, and they should not be frozen or used if they have been frozen. Once a pen is in use, many insulin pens have a limited in-use period at controlled room temperature, but the specific limits and conditions should be taken from the Awiqli labeling in your jurisdiction.
For travel, keep insulin in your carry-on rather than checked baggage to reduce temperature extremes. Avoid leaving it in a hot car, in direct sunlight, or against ice packs that could freeze the solution. Inspect the pen before each use; do not use it if you see unusual particles, clumping, or discoloration that is not consistent with the product description.
Why it matters: Temperature damage can reduce insulin reliability without obvious changes.
Side Effects and Safety
The most important safety concern with any insulin is hypoglycemia (low blood sugar). Symptoms can include sweating, shakiness, hunger, confusion, headache, irritability, or dizziness, and severe episodes can cause seizures or loss of consciousness. Risk may be higher with missed meals, unplanned exercise, alcohol intake, or when other glucose-lowering drugs are added or adjusted. Because this is a weekly basal insulin, follow-up and monitoring plans are often emphasized during initiation and dose changes.
Other possible effects include injection-site reactions (redness, pain, itching), swelling, or skin changes like lipohypertrophy if sites are not rotated. Allergic reactions are uncommon but can be serious; seek urgent care for facial swelling, breathing trouble, or widespread rash. Awiqli FlexTouch Pen should be used exactly as prescribed, and any unexpected pattern of low or high readings should be discussed with the diabetes care team rather than handled by trial-and-error adjustments.
Drug Interactions and Cautions
Many medicines can affect blood glucose or how you perceive hypoglycemia symptoms. Some drugs can increase the risk of low blood sugar, including other diabetes medications, while others may raise glucose levels (for example, certain corticosteroids). Beta-blockers may blunt typical warning signs such as tremor or palpitations, which can make lows harder to notice. Alcohol can also increase hypoglycemia risk, especially if intake is not matched with food.
Caution is often needed in people with kidney or liver impairment because insulin needs may change with altered metabolism, appetite, or concurrent illness. Pregnancy and breastfeeding considerations should be addressed with a clinician using the local label and individualized risk assessment. Always provide a complete medication list, including over-the-counter products and supplements, when a new basal insulin is started or changed.
If you want examples of how medication topics like interactions and precautions are explained in longer guides on this site, see resources such as the Clavamox Uses Safety Guide or Cephalexin Uses Dosage Guide; those guides are not about insulin but show the general education format.
Compare With Alternatives
“Basal insulin” can refer to several long-acting options that are typically dosed once daily, such as insulin glargine and insulin degludec, as well as other long-acting formulations used in specific settings. A key difference with insulin icodec is the intended once-weekly schedule, which may be convenient for some people but can also require careful planning around transitions, missed doses, and early monitoring. The best choice depends on your diabetes type, targets, prior insulin experience, and hypoglycemia risk.
Some people also use non-insulin injectables (such as GLP-1 receptor agonists) for type 2 diabetes, but these are not substitutes for basal insulin when insulin is required. If multiple injectable therapies are used, clear labeling and storage separation can reduce mix-ups.
When comparing names on a medication list, note that unrelated prescriptions on the same platform may look similar at a glance. Examples of non-diabetes products include Cyclosporine Information and Fluconazole Information; these are not alternatives to a weekly basal insulin pen.
Pricing and Access
Access to prescription insulin products depends on local authorization, a valid prescription, and pharmacy dispensing rules. When needed, prescription details can be confirmed with your prescriber before a referral is processed. Coverage varies by plan and region, and out-of-pocket costs may differ for people paying cash or without insurance, so it helps to gather your policy information and current medication list before starting any new insulin.
Other factors that can affect access include whether a prior authorization is required, whether a plan prefers a different basal insulin first, and whether the prescription specifies an exact product or allows substitution. Documentation requirements can also differ for cross-border fulfilment scenarios, and not every patient or prescription will be eligible.
Dispensing, clinical checks, and fulfilment are handled by licensed third-party pharmacies where permitted, based on jurisdictional rules. For general information about site-wide updates that may relate to access programs, you can review Promotions Information as a non-clinical reference point.
Authoritative Sources
For the most reliable details on indications, dosing conversions, missed-dose instructions, and contraindications, prioritize the official prescribing information that matches your country and the exact product package. Regulatory documents and professional organizations can also help clarify broader insulin safety topics, including hypoglycemia recognition and injection technique standards.
These sources can be helpful starting points for verification and context:
- European regulatory summary information: European Medicines Agency website
- General insulin education and safety: American Diabetes Association website
- Manufacturer background and product materials: Novo Nordisk website
When a pharmacy ships temperature-sensitive insulin, it may use prompt, express, cold-chain shipping to help protect product integrity.
This content is for informational purposes only and is not a substitute for professional medical advice.
Express Shipping - from $25.00
Shipping with this method takes 3-5 days
Prices:
- Dry-Packed Products $25.00
- Cold-Packed Products $35.00
Standard Shipping - $15.00
Shipping with this method takes 5-10 days
Prices:
- Dry-Packed Products $15.00
- Not available for Cold-Packed products
Is insulin icodec the same as rapid-acting insulin?
No. Insulin icodec is designed to act as basal insulin, meaning it provides background insulin effect over an extended period rather than covering a meal. Rapid-acting insulins are typically taken around meals to address post-meal glucose rises. Mixing up basal and rapid-acting products can increase the risk of high or low blood sugar. If you use both types, store them separately and double-check the label and dose window before each injection.
How often is a once-weekly basal insulin pen used?
A once-weekly basal insulin pen is intended to be injected one time each week, usually on the same day of the week. The specific unit dose and any transition steps from a daily basal insulin depend on the prescriber’s plan and the product labeling in your region. Because the dosing interval is longer than daily insulins, missed-dose instructions can be different. Follow the official instructions and your clinician’s directions rather than adjusting timing on your own.
What should I monitor when starting a weekly basal insulin?
Monitoring typically focuses on blood glucose patterns and signs of hypoglycemia, especially during the first weeks and after any dose changes. Many clinicians ask for more frequent checks at the start to understand fasting levels and overall trends across the week. Be alert for symptoms such as sweating, shakiness, confusion, or unusual fatigue, which can signal low blood sugar. If you use a continuous glucose monitor, review trend reports with your diabetes care team to guide safe adjustments.
What are the most important safety risks with basal insulin?
The main risk with any insulin is hypoglycemia (low blood sugar), which can be mild or severe. Severe lows may lead to falls, injuries, seizures, or loss of consciousness. Other safety concerns include allergic reactions, injection-site irritation, and skin changes like lipohypertrophy if injection sites are not rotated. Some medicines, alcohol, reduced food intake, or unexpected physical activity can increase risk. Discuss recurring lows, severe episodes, or major routine changes with a clinician promptly.
How should an insulin pen be stored during travel?
Insulin generally needs protection from temperature extremes and direct light. For travel, keep insulin in a carry-on bag and avoid leaving it in parked vehicles or near freezing conditions. Do not place the pen directly against ice packs because freezing can damage insulin. Use the specific storage instructions on the carton for unopened and in-use pens, since allowable room-temperature time can vary by product. If you suspect improper storage, ask a pharmacist how to proceed.
Can I switch from a daily basal insulin to a weekly basal insulin on my own?
No. Switching basal insulin types should be planned and supervised by a prescriber. Weekly basal insulin has different pharmacology (how the body absorbs and uses it), so conversion from daily dosing often involves a structured approach and closer monitoring at the start. Changing on your own can increase the risk of hypoglycemia or persistent hyperglycemia. If you are interested in switching, bring your current insulin details, recent glucose readings, and any hypoglycemia history to a clinician for review.
What should I ask my clinician before using a weekly insulin pen?
Helpful questions include: what diabetes type and goals the plan is targeting; how the starting dose was chosen; how and when to check glucose during the first weeks; what to do if you miss a scheduled dose; and how to handle illness, appetite changes, or increased activity. It can also help to ask about interactions with other glucose-lowering medicines and alcohol. Finally, request a demonstration of injection technique and how to confirm the correct dose on the pen.
Rewards Program
Earn points on birthdays, product orders, reviews, friend referrals, and more! Enjoy your medication at unparalleled discounts while reaping rewards for every step you take with us.
You can read more about rewards here.
POINT VALUE
How to earn points
- 1Create an account and start earning.
- 2Earn points every time you shop or perform certain actions.
- 3Redeem points for exclusive discounts.
You Might Also Like
Related Articles
Why Is Ozempic So Expensive? Pricing Factors Explained
Key Takeaways Price varies because list price differs from what payers actually pay. Insurance design (deductibles, coinsurance, formularies) often drives your out-of-pocket amount. High demand and limited competition can keep…
Zepbound Pill Clarified: Injection Reality and Oral Research
Key Takeaways Current form: Zepbound is an injectable medicine, not a tablet. Search intent: “pill” usually means convenience, not a new product. Dosing language: labels use stepwise titration and maintenance…
Lancets For Blood Sugar Testing: Selection And Safety Tips
OverviewFingerstick blood glucose checks rely on small, sharp tools and consistent technique. In most home setups, a spring-loaded lancing device uses lancets to puncture skin and produce a drop of…
SGLT2 Inhibitors Explained: Uses, Risks, and Examples
Key Takeaways These medicines lower blood sugar by acting in the kidneys. Some are also labeled for heart failure or chronic kidney disease. Drug names include dapagliflozin, empagliflozin, and canagliflozin.…