Key Takeaways
- Signal overview: hormone, receptor, cellular response.
- Receptor activation: tyrosine kinase and phosphorylation cascades.
- Metabolic outcomes: glucose uptake and storage.
- Pathology: defects contribute to insulin resistance.
Cells manage energy by responding to hormones with precision. In this context, insulin signaling coordinates glucose uptake, lipid metabolism, and protein synthesis across tissues. Understanding the sequence makes complex diagrams easier to interpret and apply to clinical cases.
Insulin Signaling: Core Concepts and Cellular Effects
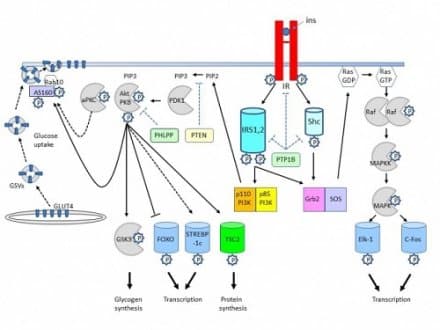
Insulin’s primary job is to help cells use and store nutrients. Clinically, this means lowering circulating glucose while promoting glycogen, lipid, and protein synthesis. At the cellular level, insulin binds its receptor and triggers phosphorylation cascades. These cascades recruit transporters like GLUT4 to the membrane in muscle and adipose tissue, enabling rapid glucose entry.
Downstream, several nodes coordinate different outcomes. PI3K–Akt signaling supports glucose transport and glycogen synthesis, while mTOR (a growth regulator) favors protein synthesis. In the liver, insulin suppresses gluconeogenesis (glucose production) and promotes glycogen formation. This integrated response balances energy needs across organs and time.
For a broader disease context and therapy landscape, see Common Diabetes Medications for mechanisms that interact with these pathways in practice: Common Diabetes Medications.
From Hormone Release to Receptor Binding
Insulin is produced by pancreatic beta cells as preproinsulin, processed to proinsulin, and then cleaved into insulin and C-peptide before secretion. Glucose entry via GLUT2 raises ATP, closes K+ channels, and opens Ca2+ channels, driving vesicle fusion. This process links nutrient levels to hormone output in minutes.
A common question is what organ in the human body produces insulin. The source is the pancreas, specifically the islets of Langerhans. For anatomical context and function, see this primer, which outlines cellular localization and roles: Insulin-Producing Organ. For how manufacturing mirrors biology, review this overview of recombinant production for structure background: How Synthetic Insulin Is Made.
Receptor Architecture and Activation
The insulin receptor sits as a dimer at the cell surface and spans the membrane. When insulin binds, the receptor’s intracellular tyrosine kinase (enzyme that adds phosphate groups) becomes active. It phosphorylates IRS proteins, which relay the signal to PI3K and other effectors. This switching logic is conserved across tissues, with context-specific outputs.
According to a detailed NCBI review, the receptor is a tyrosine kinase that auto-phosphorylates after ligand binding, enabling adaptor recruitment; this mechanism underpins metabolic effects (NCBI Bookshelf overview). For clinical learners, recognizing kinase activation clarifies why mutations or serine phosphorylation can derail downstream responses.
Tyrosine Kinase Cascades and Metabolic Outputs
Activated receptor and IRS proteins recruit PI3K, generating PIP3 at the membrane. PIP3 brings PDK1 and Akt into position; Akt then phosphorylates targets that drive GLUT4 translocation, glycogen synthesis via GSK3 inhibition, and lipogenesis through SREBP pathways. Parallel Ras–MAPK signaling influences growth and gene expression, more prominent in mitogenic contexts.
These branches often interact. For example, Akt inhibits FoxO transcription factors to reduce hepatic gluconeogenic gene expression. Meanwhile, mTORC1 integrates amino acid status with growth signaling. This cross-talk explains why nutrient state, inflammation, and exercise modify the cellular response to a single insulin pulse.
For an in-depth pharmacologic counterpoint affecting AMPK and hepatic output, see this analysis, which complements receptor-driven cascades: Metformin Mechanism.
Structural Insights and Isoforms
The insulin receptor structure features alpha subunits that bind insulin and beta subunits with kinase domains. Disulfide bonds stabilize the dimer, while glycosylation modulates trafficking and stability. Splice variants (IR-A, IR-B) differ slightly in their extracellular domains, which may influence ligand preference and tissue distribution.
Understanding these architectural details helps interpret differences in metabolic versus mitogenic responses. For instance, variant expression patterns may shape growth signals in fetal tissues versus metabolic control in adult liver. A concise physiology summary from NIH emphasizes how receptor design matches function across organ systems (physiology of insulin secretion).
Mapping the Pathway: Steps and Cross-Talk
Many learners prefer flow charts to track sequence. A practical way is to memorize anchor points: hormone release, receptor binding, kinase activation, adaptor phosphorylation, lipid second messengers, and terminal effectors. Once these are clear, the diagram becomes a map of interacting lanes rather than a maze.
For study focus, outline the insulin signaling pathway steps as numbered blocks. Start with ligand binding and receptor autophosphorylation. Continue through IRS phosphorylation, PI3K activation, PIP3 generation, PDK1/Akt assembly, and end with GLUT4 translocation, glycogen synthesis, and gene regulation. Build a personal schematic or flashcards to reinforce order and connections. For a curated set of disease-related mechanisms, explore this topical hub: Diabetes Guides.
Tip: Convert complex figures into a three-column list—event, mediator, outcome—and rewrite in your own words. This technique reduces cognitive load when revisiting larger diagrams or slide decks.
Insulin in Health and Disease
When signaling is intact, tissues respond briskly to meals and fasting. Skeletal muscle handles most postprandial glucose uptake, while adipose tissue stores triglycerides and releases them during fasting. The liver switches between storage and production, guided by hormone levels and nutrient status. This flexible system protects the brain and maintains steady plasma glucose.
Chronic overnutrition, inflammation, or genetics can impair the cascade. The result is insulin resistance, where normal hormone levels yield weaker responses. Over time, the pancreas compensates by secreting more insulin, sometimes exhausting beta cells. For real-world contributors beyond biology, see this review of non-traditional exposures that affect metabolic risk: Environmental Hazards and Diabetes. For tissue-preserving strategies relevant to exocrine-endocrine interactions, see Pancreatitis and Diabetes for preservation-focused insights.
Therapies may target multiple nodes. Metformin dampens hepatic glucose output and influences cellular energetics. Incretin-based agents enhance glucose-dependent insulin secretion. For a balanced look at two modern incretin agents and their distinct pharmacology, review Orforglipron vs Tirzepatide, and for GLP-1/GIP comparisons in weight management contexts see Tirzepatide vs Semaglutide. For cross-over benefits outside diabetes, see Metformin’s Potential to understand repurposing hypotheses.
Counterbalance: Glucagon, IGF, and Nutrient State
Insulin works alongside glucagon to stabilize blood glucose across fasting and feeding. The pair—often discussed as insulin and glucagon—adjust liver output and tissue uptake in opposite directions. During fasting, glucagon rises to mobilize hepatic glucose. After meals, insulin predominates and promotes storage. The ratio, not just absolute levels, often determines net metabolic outcome.
Insulin-like growth factors (IGFs) share structural features and signaling nodes with insulin. IGF-1 receptors prefer growth-related signaling through MAPK pathways, while insulin’s metabolic effects rely more on PI3K–Akt. Nutrient availability fine-tunes both systems. Appreciating this overlap explains why growth states, stress, and illness can modify apparent insulin responsiveness. For a broad list of medication classes used in clinics, see the catalog overview for educational browsing: Diabetes Medications Category.
Study Aids: From Slides to Simple Summaries
Efficient review tools help consolidate knowledge. Convert a dense lecture deck into a concise outline with three layers: receptor events, second messengers, and cellular outcomes. Next, add a one-page summary for quick recall before exams or clinics. Revisit the outline when you encounter new therapies or biomarkers to see where they act.
If you prefer visual learning, sketch a minimal pathway with six boxes and arrows. Add color only when you are confident with the base. Then annotate where particular drug classes act. Pair this with short readings on physiology to keep fundamentals fresh alongside new research. For endocrine physiology grounding aligned with clinical practice, see the American Diabetes Association’s standards for contextual guidance (ADA clinical standards).
Note: Signaling cascades vary by tissue, timing, and nutrient state. Interpret any single diagram as a template, not a rigid rule set.
Recap
Insulin coordinates nutrient handling across tissues through receptor-initiated kinase cascades. Receptor activation, adaptor phosphorylation, and downstream branches like PI3K–Akt and MAPK produce predictable metabolic effects. Disruptions to this choreography can impair glucose control and drive chronic disease. Reinforcing core steps, and knowing where therapies act, makes the pathway clearer and clinically useful.
This content is for informational purposes only and is not a substitute for professional medical advice.