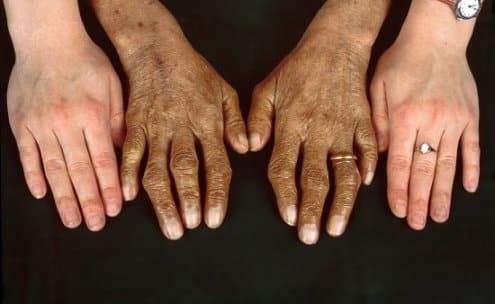
What is Hemochromatosis (Bronze Diebetes)?
Hemochromatosis, also known as bronze diabetes, is a disorder that prompts the body to absorb and accumulate excess iron.
The intestines absorb a specific amount of iron from an individual’s food intake, with the amount carefully calibrated according to how much iron the body needs. This gets distributed throughout the entire body to assist the hemoglobin in blood cells in carrying oxygen to organs and tissues.
With bronze diabetes, the body absorbs more iron than the body needs. The body has no mechanism to remove the excess iron, so it gets stored in joints and organs— most particularly in the liver, heart, and pancreas. The excessive accumulation of iron can damage tissues and organs, which may lead to life-threatening conditions. This is why hemochromatosis is referred to as iron overload disorder.
Signs and Symptoms
Although hereditary hemochromatosis is congenital, most individuals don’t exhibit symptoms until later in life. Men are known to show signs after the age of 40 and women after the age of 60. For women, symptoms are most likely to develop after menopause since menstruation and pregnancy eliminate excess iron in the body.
Some early signs include:
- Extreme tiredness (fatigue)
- Joint pain
- Abdominal pain
- Muscle weakness
- Loss of sex drive
- Weight loss
- Hair loss
More serious symptoms include:
- Diabetes
- Heart abnormalities
- Liver disease (cirrhosis)
- Skin discoloration (bronze or gray)
- Memory Haziness
The severity of the symptoms is affected by lifestyle choices and environmental factors such as iron intake, alcohol use, and other medical conditions.
Hemochromatosis Types and Causes
There are two main types of Hemochromatosis: primary hemochromatosis and secondary hemochromatosis.
Primary Hemochromatosis, also referred to as Hereditary Hemochromatosis, is caused by a mutation in the gene that is responsible for regulating the amount of iron the body absorbs after meals. This is inherited and passed on from parents to children.
Secondary hemochromatosis is the result of other medical conditions, such as thalassemia (an inherited blood disorder), anemia, chronic alcoholism, and other disorders.
There are four types of hereditary hemochromatosis, which are classified according to the age of onset, mode of inheritance, and genetic cause.
Type 1, the most common form of the disorder, begins to develop symptoms in late adulthood. Men usually start showing symptoms between the ages of 40 and 60, and women after menopause.
Hemochromatosis type 1 is inherited by an autosomal recessive pattern in the mutation of a gene called HFE. HFE has two common mutations, C28Y and H63D. Every individual inherits one HFE gene from each parent, and should one inherit 2 abnormal genes, then the individual is likely to develop hemochromatosis.
Type 2 hemochromatosis is called a juvenile-onset disorder because the symptoms start appearing in childhood. Over time, this causes a decreased or non-existent secretion of sex hormones. Males may undergo delayed puberty or any other symptoms related to a shortage or absence of sex hormones. Females may begin menstruation normally that stops after a few years. Type 2 is inherited by an autosomal recessive pattern in either the HJV or HAMP gene.
Type 3 hemochromatosis is characterized by the onset of symptoms developing in the ages between Type 1 and Type 2. Individuals with Type 3 hemochromatosis usually develop symptoms during early adulthood years, usually before the age of 30. This is caused by mutations in the inherited TFR2 gene, which gets passed on through an autosomal recessive pattern.
Type 4 hemochromatosis is similar in the onset time of symptoms with Type 1. They are distinguished from one another because Type 4 is caused by a mutation in a different gene that is passed on by the autosomal dominant inheritance pattern. Type 4 is caused by an abnormality in the SLC40A1 gene.
Since Type 1, 2, and 3 Hereditary Hemochromatosis is passed on through the autosomal recessive pattern, both parents must have one copy of the mutated gene for an individual to have hemochromatosis. Some people who have only one copy of the defective gene are called “carriers”, and there is a chance of passing this onto their child.
These genes produce proteins that regulate the absorption, transport, storage, and usage of iron in the body. With the mutations in these genes, their ability to perform critical controls in the body’s management of iron leads to excessive iron accumulation.
Diagnosis
There is an identified difficulty in diagnosing Hemochromatosis since all its symptoms can be attributed to other (or in of itself already are) existing medical conditions. Iron Overload Disorder is easy to overlook if the medical attention given to the patient is concentrated only in treating the diseases present in the individual that are due to hemochromatosis.
Some people have asymptomatic hemochromatosis and don’t exhibit any symptoms at all aside from an elevated iron levels in their blood.
To diagnose iron overload, two key blood tests must be done:
Serum transferrin saturation. This test measures how much iron is bound to the protein called transferrin in your body. Transferrin is responsible for carrying iron in the blood. Transferrin saturation values of 45% and above are considered too high.
Serum ferritin. This test measures how much iron is stored in the liver. When the serum transferrin saturation test render results that are above normal, the doctor will check for the serum ferritin.
These tests alone do not prove the existence of hemochromatosis since elevated ferritin can be caused by other medical conditions. Individuals with test results that reveal an abnormally high amount of iron should undergo genetic testing to confirm the mutations.
Additional tests include liver function tests and liver biopsy to check the presence of iron and evidence of liver damage, especially scarring or cirrhosis. An MRI can also be used to determine the degree of iron overload in the liver. Testing for gene mutations is also recommended, under the guidance of a doctor or genetic counselor.
Genetic testing is also recommended for the parents, siblings, and children of an individual who has been diagnosed with hemochromatosis. If the mutation exists only in one parent, then children may skip testing.
Treatment
Phlebotomy
Hereditary Hemochromatosis is treated by reducing iron levels to normal, which can be done by removing blood from the body (phlebotomy) regularly.
The frequency, amount of blood to be removed, and longevity of the entire process are determined according to by age, overall health, the severity of the iron overload, and other medical conditions one might be experiencing. It may take a year or more to return iron levels to normal.
Initially, a pint (470 milliliters) of blood may be taken once or twice a week, the process similar to donating blood. A needle is inserted into a vein the arm, and the blood flows from the needle into a tube that’s attached to a blood bag.
Once iron levels have resumed to normal, the frequency of removing blood is lessened, and some people may maintain normal iron levels without having any blood taken. The maintenance treatment depends on how rapid the rate of iron accumulation is in the body.
Phlebotomy cannot improve any existing conditions including cirrhosis or joint pain. These conditions have their own treatments that must also be undergone immediately.
Chelation
For individuals that cannot undergo phlebotomy because of medical conditions such as anemia and other heart complications, the doctor may remove a medication that is either taken orally as a pill or injected into the body to remove excess iron. The medicine binds with excess iron to dispose of it through the urine or stool through a process called chelation.
Conclusion
Hemochromatosis, or iron overload disorder, is a tricky medical condition that may hide under the guise of diseases that are only caused by it. Hemochromatosis must be identified as soon as possible. Early identification leads to early treatment, which can help alleviate the symptoms of tiredness, pain, and skin darkening.
Early treatment can prevent its aggravation to serious conditions such as liver and heart complications, and diabetes. It may also slow down the progression of the disease, and in the best case, reverse it. Prevention is always better than cure.
Doctor’s Recommendation
For patients concerned about rare illnesses, it is recommended to have a complete metabolic panel with iron studies every 1 to 2 years. This frequency is sufficient to detect the disease early and manage it effectively.
In the most common form of these illnesses, damage typically takes decades to begin, so there is no immediate cause for concern. While ferritin levels can be elevated in inflammatory conditions, they can usually be interpreted accurately based on symptoms and context. If there is still uncertainty about ferritin levels, physicians may order soluble transferrin receptor (sTfR) tests to differentiate. However, this additional testing is rarely necessary.
Regular monitoring and context-based interpretation will help manage and detect any potential issues effectively.
—