Please note: a valid prescription is required for all prescription medication.
Wegovy product overview: uses, dosing, and safety
Start 2026 with savings: Use code SAVE10 for 10% OFF all RX meds. Jan–1 Mar. Ozempic from Canada and Mounjaro Vial not included. Offer valid until March 1st. Coupon code cannot be combined with other offers. For products with “Bulk Savings”, the discount will be applied to the regular price for 1 unit. Maximum allowable quantity equal to a 90 day supply per single order.
Price range: $399.99 through $529.99
You save


Wegovy is a prescription, once-weekly injectable medicine (semaglutide) used for chronic weight management with nutrition and activity changes. This page summarizes how it works, how the pen is used, and key safety topics to review with a clinician. Some patients explore US delivery from Canada when discussing cross-border logistics for ongoing therapy.
What Wegovy Is and How It Works
This medicine belongs to a class called GLP-1 receptor agonists. GLP-1 (glucagon-like peptide-1) is a gut hormone that affects appetite regulation and digestion. By activating GLP-1 receptors, semaglutide can help people feel full sooner, reduce hunger between meals, and slow stomach emptying. These effects can support lower calorie intake when paired with lifestyle changes.
Weight management treatment works best when goals are clear and follow-up is routine. Our role is coordinating prescription referral for eligible patients. Ongoing monitoring is still done by the prescribing clinician, who can assess response, tolerability, and safety over time.
Because GLP-1 signaling also affects glucose (blood sugar) pathways, some people notice changes in glucose readings, especially if they also use diabetes medicines. Slower stomach emptying can influence how quickly carbohydrates are absorbed after meals, and it may affect the absorption timing of some oral medications. For that reason, medication review is an important part of starting therapy.
Individual benefits and risks vary by medical history. When required, prescription details are confirmed with the prescriber. Reviewing the official labeling can also clarify contraindications, boxed warnings, and monitoring recommendations relevant to your situation.
Who It’s For
This treatment is indicated for chronic weight management in specific populations defined in the prescribing information. In general terms, it may be used in adults with obesity, or in adults who are overweight and also have at least one weight-related condition. It is also indicated for certain adolescents aged 12 years and older with obesity, under clinician supervision and with family support.
Eligibility is based on clinical criteria, not appearance goals. Condition hubs like Obesity Hub and Overweight Hub can help patients understand how products are commonly organized by indication, while Weight Management lists related prescription options by category.
This medicine is not appropriate for everyone. People with a personal or family history of medullary thyroid carcinoma (MTC) or with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2) should not use semaglutide products used for weight management. It is also contraindicated in patients with a known serious hypersensitivity (allergic reaction) to semaglutide or any component of the product.
Pregnancy planning should be discussed before starting, because intentional weight loss therapies are generally not recommended during pregnancy. People with a history of pancreatitis, gallbladder disease, severe gastrointestinal disease, or significant kidney issues should discuss added precautions. If you have diabetes, especially with eye disease (diabetic retinopathy), your clinician may recommend closer monitoring.
Dosage and Usage
Dosing is typically started at a low level and increased in steps to improve tolerability, especially for gastrointestinal side effects. The injection is given subcutaneously (under the skin) on the same day each week, at any time of day, with or without meals. Follow the label and prescriber instructions for missed doses, because recommendations can differ based on how close you are to the next scheduled dose.
Many patients find it helpful to read a week-by-week overview before the first injection. For practical orientation, see Wegovy Doses Guide and the broader reference Semaglutide Dosage Chart, then confirm details with the prescribing clinician.
Quick tip: Rotate injection areas and keep a simple calendar reminder.
Common injection sites include the abdomen, thigh, or upper arm. Choose intact skin and avoid areas that are bruised, tender, hard, scarred, or affected by stretch marks. If you are thinking about a specific wegovy injection site, the goal is usually consistency and rotation rather than finding a single “best” spot. Do not share injection devices, even within the same household, because sharing can transmit infections.
The pen is designed to deliver a single dose. Use a sharps container for disposal and follow local disposal rules. If you have tremor, vision limitations, or needle anxiety, a pharmacist or clinician can review safe handling steps and confirm you understand the instructions before you start.
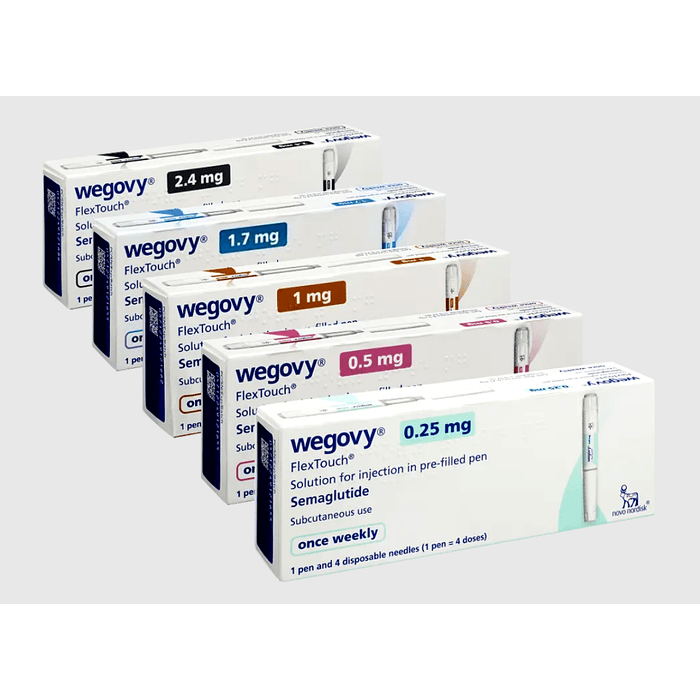
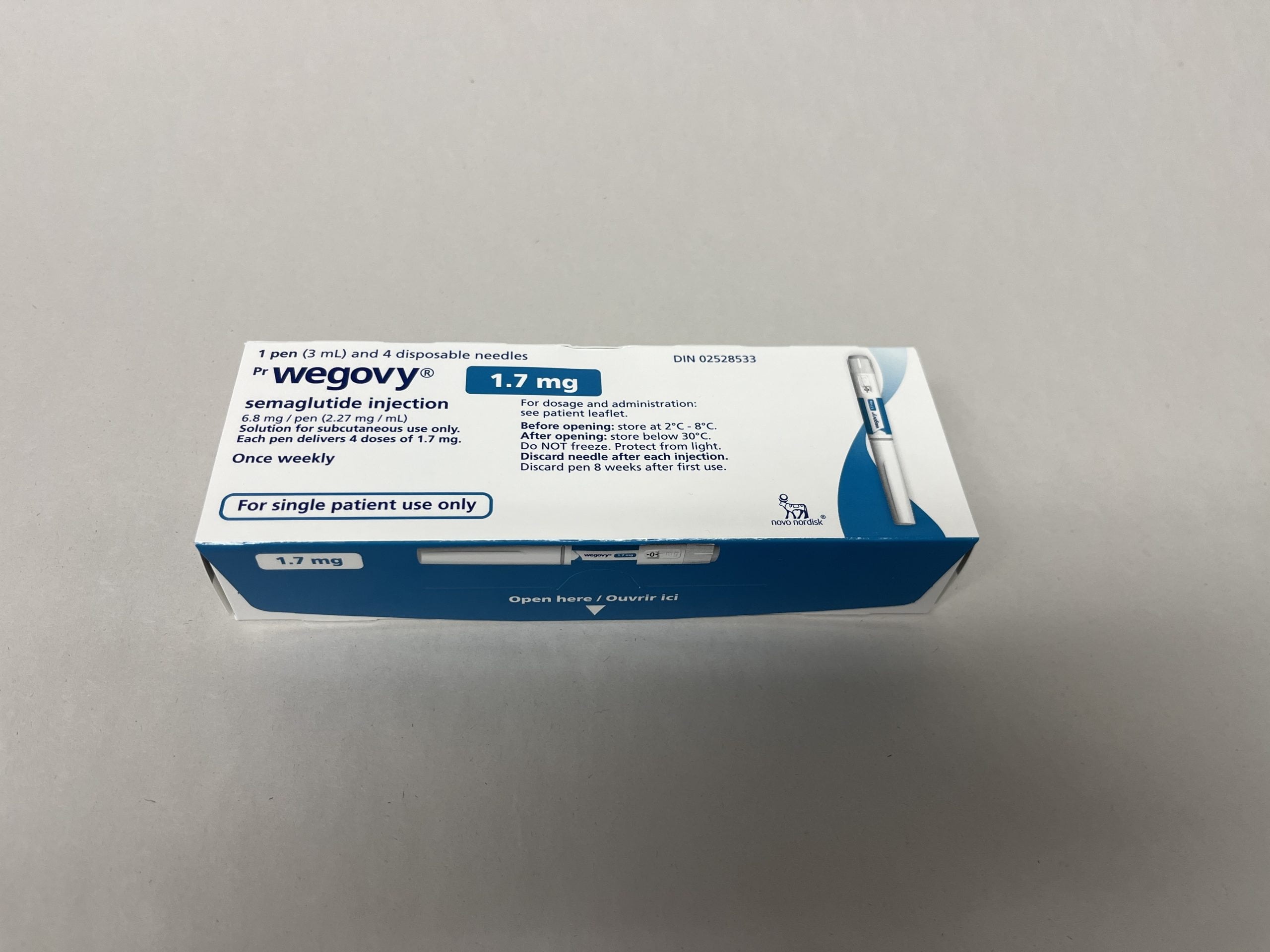
Strengths and Forms
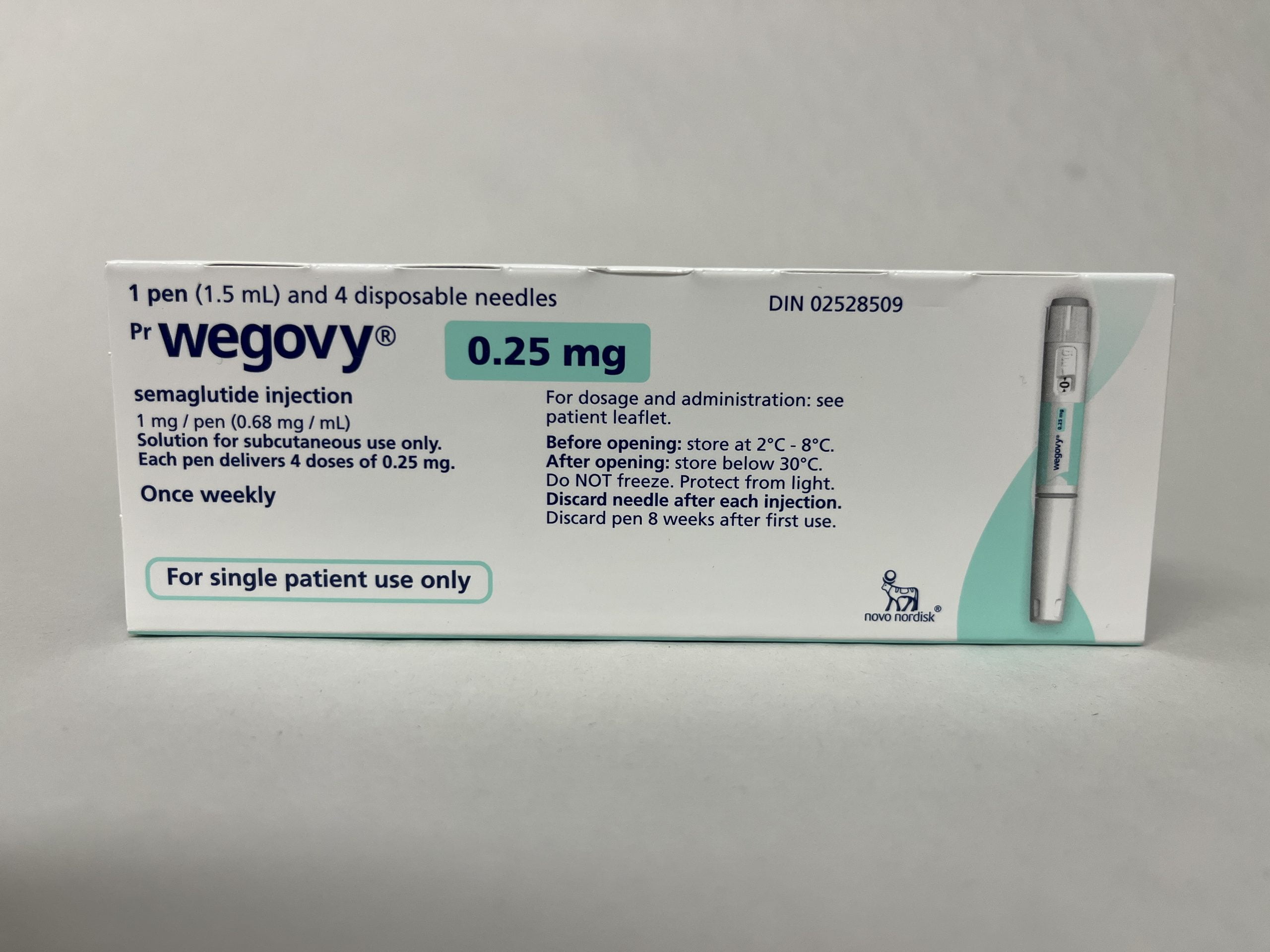
This product is supplied as prefilled, single-dose injection pens in multiple dose strengths. Your prescription will specify the dose strength used for your current step, and availability can vary by region and pharmacy supply. Wegovy titration commonly progresses through several strengths before reaching a maintenance dose, but the prescriber may pause or adjust progression based on tolerability.
The medicine is administered subcutaneously using the pen’s built-in delivery system. Unlike multi-dose pens, single-dose presentations are intended for one injection and then disposal. Patients should verify the carton and pen labeling each time to confirm the dose strength matches the current prescription.
| Common pen strengths | Typical role in titration |
|---|---|
| 0.25 mg | Starting step |
| 0.5 mg | Early escalation step |
| 1.0 mg | Mid escalation step |
| 1.7 mg | Late escalation step |
| 2.4 mg | Maintenance step for many patients |
If a pen looks damaged, has been stored incorrectly, or the solution appears discolored or cloudy, do not use it. Confirm next steps with a pharmacist or the prescribing clinician.
Storage and Travel Basics
Proper storage helps maintain medication stability. Keep Wegovy refrigerated at 2°C to 8°C (36°F to 46°F) when possible, and protect it from excessive heat and light. Do not freeze the pens, and do not use them if they have been frozen. Keep the product in the original carton until use to reduce light exposure.
The labeling allows limited room-temperature storage in many cases. In general, pens may be kept at 8°C to 30°C (46°F to 86°F) for up to 28 days, but the label should be followed exactly. If you are unsure how long a pen has been out of the refrigerator, do not guess; confirm with a pharmacist.
For travel planning, pack supplies so they are easy to inspect and protected from crushing. Consider carrying a copy of the prescription and keeping temperature-sensitive medicines in your carry-on rather than checked luggage. The guide Travel With Ozempic covers practical temperature and packing concepts that are also relevant to weekly GLP-1 injections.
When you arrive, store pens promptly according to the label. If a dose is due during travel, plan a clean injection environment and a safe place to store used sharps until disposal is available.
Side Effects and Safety
Like other GLP-1 receptor agonists, Wegovy commonly causes gastrointestinal effects, especially during dose escalation. Nausea, vomiting, diarrhea, constipation, stomach pain, and decreased appetite are frequently reported. Headache, fatigue, dizziness, reflux symptoms, and injection-site reactions can also occur. Many people find side effects improve as the body adjusts, but symptoms that persist or limit hydration should be discussed with a clinician.
Why it matters: Vomiting or diarrhea can lead to dehydration and kidney strain.
Serious risks are uncommon but important to recognize. Semaglutide weight-management products carry a boxed warning about thyroid C-cell tumors observed in rodents; the relevance to humans is unknown, and it is contraindicated in patients with MTC or MEN 2. Seek urgent care for severe abdominal pain that may radiate to the back (possible pancreatitis), symptoms of gallbladder problems (such as persistent right upper abdominal pain with fever or jaundice), or signs of a serious allergic reaction (swelling of face or throat, trouble breathing, widespread rash).
If you have diabetes and take insulin or a sulfonylurea, low blood sugar risk can increase and may require closer monitoring. Some patients should also be monitored for mood changes; report new or worsening depression, anxiety, or suicidal thoughts promptly. Your clinician may periodically review weight trend, heart rate, kidney function (especially if significant GI symptoms occur), and any changes in vision if you have diabetes.
Drug Interactions and Cautions
This medicine slows gastric emptying, which can change the timing of absorption for some oral drugs. For most medications, this does not require changes, but it can matter for therapies where small blood-level changes are clinically important. Tell your clinician about all prescription drugs, over-the-counter products, and supplements, and mention any history of severe reflux, gastroparesis (delayed stomach emptying), or bariatric surgery.
Extra caution is needed when semaglutide is used alongside insulin or insulin secretagogues (such as sulfonylureas), because hypoglycemia can occur. Clinicians often give monitoring instructions for home glucose checks when relevant. Alcohol use can also complicate GI symptoms and glucose control for some people, so it is worth discussing patterns of intake during medication review.
Avoid combining GLP-1 receptor agonists unless specifically directed, since overlapping effects can increase side effects without added benefit. If you take other weight-management drugs, your prescriber should review whether combination therapy is appropriate and evidence-supported for your goals. Always follow label precautions for patients with prior pancreatitis, gallbladder disease, kidney disease, and diabetic eye disease.
Before procedures that require fasting or sedation, tell the procedural team you use a weekly GLP-1 medicine. They may give instructions to reduce aspiration risk based on current clinical guidance and your personal situation.
Compare With Alternatives
Several prescription options are used for chronic weight management, and differences matter for dosing frequency, mechanism, and labeled indications. One alternative is tirzepatide, a dual GIP/GLP-1 receptor agonist, which is marketed for weight management as Zepbound Product. Another option is liraglutide (a daily GLP-1 receptor agonist injection), which some patients use when weekly therapy is not a fit.
Semaglutide is also available under other brand labeling for type 2 diabetes, such as Ozempic Semaglutide Pens (different labeled indication and dosing). Oral semaglutide is available for diabetes as well, which can be relevant for patients who cannot use injections, though the labeling and clinical goals differ.
If you want a mechanism-focused comparison, resources like Tirzepatide Vs Semaglutide and Saxenda Vs Ozempic can help frame questions to bring to your clinician. The best choice depends on medical history, contraindications, tolerability, and practical factors such as dosing schedule and monitoring needs.
Switching between therapies should be clinician-guided. Do not assume dose equivalence across products, even within the same drug class, because titration schedules and approved uses can differ.
Pricing and Access
Access varies by plan design, local availability, and clinical documentation. Wegovy requires a prescription, and many insurers use prior authorization for weight-management medicines. Coverage criteria may consider BMI thresholds, weight-related conditions, previous weight-management attempts, and safety factors. If coverage is denied, the prescriber may be able to submit additional documentation or discuss alternatives that align with your health needs.
For patients without insurance, cash-pay planning often involves comparing pharmacy policies, confirming the exact dose strength, and understanding what supplies are included. The guide Out Of Pocket Cost reviews common budgeting and verification steps for GLP-1 therapies without quoting specific prices. Browsing Weight Management Articles can also help you understand how different medications are typically positioned.
Dispensing is handled by licensed third-party pharmacies where permitted. Depending on eligibility and jurisdiction, cross-border fulfilment considerations may apply and can require additional review of prescription details and patient information.
To reduce delays and mix-ups, it helps to keep a current medication list, allergy history, and recent diagnosis details available for your clinician’s records. Do not start, stop, or change dose timing without prescriber guidance, especially if you also use medicines that affect blood sugar.
Authoritative Sources
For the most reliable details on indications, contraindications, and administration, consult primary sources and confirm questions with a licensed clinician. Official labeling is the best reference for storage limits, missed-dose instructions, and warnings that may apply to your medical history.
For the FDA-approved prescribing information, see this official label resource: FDA Prescribing Information PDF.
For manufacturer materials and medication guides, use the official product site resources: Manufacturer Medication Information.
For patient-friendly education on semaglutide injections, see a national library reference: MedlinePlus Semaglutide Injection.
Temperature-sensitive medicines may be packed for prompt, express, cold-chain shipping to help protect stability.
This content is for informational purposes only and is not a substitute for professional medical advice.
Express Shipping - from $25.00
Shipping with this method takes 3-5 days
Prices:
- Dry-Packed Products $25.00
- Cold-Packed Products $35.00
Standard Shipping - $15.00
Shipping with this method takes 5-10 days
Prices:
- Dry-Packed Products $15.00
- Not available for Cold-Packed products
What is Wegovy used for?
Wegovy is a prescription medicine used for chronic weight management in specific populations defined by the product labeling. It is used together with reduced-calorie nutrition and increased physical activity, not as a stand-alone approach. It contains semaglutide, a GLP-1 receptor agonist that can reduce appetite and slow stomach emptying. A clinician determines whether it is appropriate based on factors such as BMI, weight-related health conditions, contraindications, and overall risk profile.
How does the Wegovy pen work for weekly injections?
The Wegovy pen is a prefilled, single-dose device designed to deliver one subcutaneous (under-the-skin) injection. The prescription specifies the dose strength for your current step. In general, patients inject on the same day each week and follow the label for missed-dose instructions. Because it is single-dose, the pen is disposed of after use in a sharps container. If any step is unclear, a pharmacist or clinician can demonstrate handling and review the manufacturer’s instructions.
Where are recommended injection sites for Wegovy?
Common injection areas for weekly GLP-1 medicines include the abdomen, thigh, or upper arm. The goal is to inject into clean, intact skin and rotate locations to reduce irritation. Avoid skin that is bruised, tender, hard, scarred, or affected by active rash. Rotation can be as simple as alternating left and right sides or moving at least an inch from the prior spot within the same general area. Follow the pen instructions and ask your clinician if you have skin conditions or concerns about technique.
What side effects should I monitor while taking Wegovy?
Gastrointestinal symptoms are common, especially when the dose is being increased. Nausea, vomiting, diarrhea, constipation, and abdominal discomfort can occur and may affect hydration. Contact a clinician promptly for severe or persistent symptoms, signs of dehydration, or if you cannot keep fluids down. Seek urgent care for severe abdominal pain that may suggest pancreatitis, symptoms of gallbladder disease (such as persistent right upper abdominal pain or jaundice), or signs of a serious allergic reaction. Report mood changes or suicidal thoughts right away.
Can Wegovy interact with other medications?
Semaglutide can slow gastric emptying, which may change the timing of absorption for some oral medications. This is usually manageable, but it can be important for medicines with a narrow therapeutic window. If you use insulin or a sulfonylurea, low blood sugar risk can increase, so clinicians often recommend additional glucose monitoring or adjustments to the diabetes regimen. Provide a complete medication and supplement list to your prescriber, and tell them about any history of gastroparesis or significant gastrointestinal disease.
What should I ask my clinician before starting Wegovy?
Helpful questions include whether you meet the labeled criteria for chronic weight management, what titration schedule they plan to use, and what side effects are most likely during dose escalation. Ask about contraindications such as personal or family history of medullary thyroid carcinoma or MEN 2, and how pregnancy planning affects treatment. If you have diabetes, discuss glucose monitoring, hypoglycemia risk with other medicines, and whether eye monitoring is needed for diabetic retinopathy. Also ask for clear instructions on missed doses and storage during travel.
How should Wegovy be stored at home and during travel?
Store pens in the refrigerator when possible and protect them from light. Do not freeze the medication, and do not use it if it has been frozen. The labeling allows limited room-temperature storage within a defined range for a limited number of days, so it’s important to follow the package instructions exactly. For travel, keep the medicine in your carry-on to avoid temperature extremes in checked baggage, and pack a sharps container or an approved alternative for used devices. If you are unsure whether storage limits were exceeded, ask a pharmacist before using the pen.
Rewards Program
Earn points on birthdays, product orders, reviews, friend referrals, and more! Enjoy your medication at unparalleled discounts while reaping rewards for every step you take with us.
You can read more about rewards here.
POINT VALUE
How to earn points
- 1Create an account and start earning.
- 2Earn points every time you shop or perform certain actions.
- 3Redeem points for exclusive discounts.
You Might Also Like
Related Articles
Zepbound Pill Clarified: Injection Reality and Oral Research
Key Takeaways Current form: Zepbound is an injectable medicine, not a tablet. Search intent: “pill” usually means convenience, not a new product. Dosing language: labels use stepwise titration and maintenance…
Lancets For Blood Sugar Testing: Selection And Safety Tips
OverviewFingerstick blood glucose checks rely on small, sharp tools and consistent technique. In most home setups, a spring-loaded lancing device uses lancets to puncture skin and produce a drop of…
SGLT2 Inhibitors Explained: Uses, Risks, and Examples
Key Takeaways These medicines lower blood sugar by acting in the kidneys. Some are also labeled for heart failure or chronic kidney disease. Drug names include dapagliflozin, empagliflozin, and canagliflozin.…
Out Of Pocket Cost For GLP-1 Medications: Planning Tips
Key Takeaways Costs vary by drug, indication, and dose form Cash-pay totals include more than the pen Programs exist, but eligibility is limited Be cautious with compounded versions and unverifiable…