Recent trials suggest an artificial pancreas can help automate glucose control in adults with type 2 diabetes. These systems combine a glucose sensor, an insulin pump, and an algorithm to adjust dosing. Results vary by user, but the approach may reduce daily decision load and improve time in range.
Key Takeaways
- Closed-loop systems adjust insulin using real-time glucose data.
- Type 2 trials show improved control and fewer lows.
- Training, follow-up, and troubleshooting remain essential.
- Coverage and device availability differ by region and plan.
Trial Overview and Outcomes
Clinical studies tested these systems in adults using basal-bolus insulin therapy. In general, participants had more time in target range and fewer overnight hypoglycemia (low blood sugar) events. Importantly, daily self-management burdens decreased because the algorithm made frequent adjustments. These findings align with growing evidence from inpatient and outpatient studies using standardized endpoints.
Early work on the artificial pancreas for type 2 diabetes focused on safety, usability, and real-world adherence. Researchers tracked mean glucose, glycemic variability (blood sugar swings), and device wear time. Several studies reported high satisfaction with fewer manual corrections. For background on closed-loop basics and core components, see Automating Glucose Control for foundational concepts and definitions.
How the artificial pancreas Works
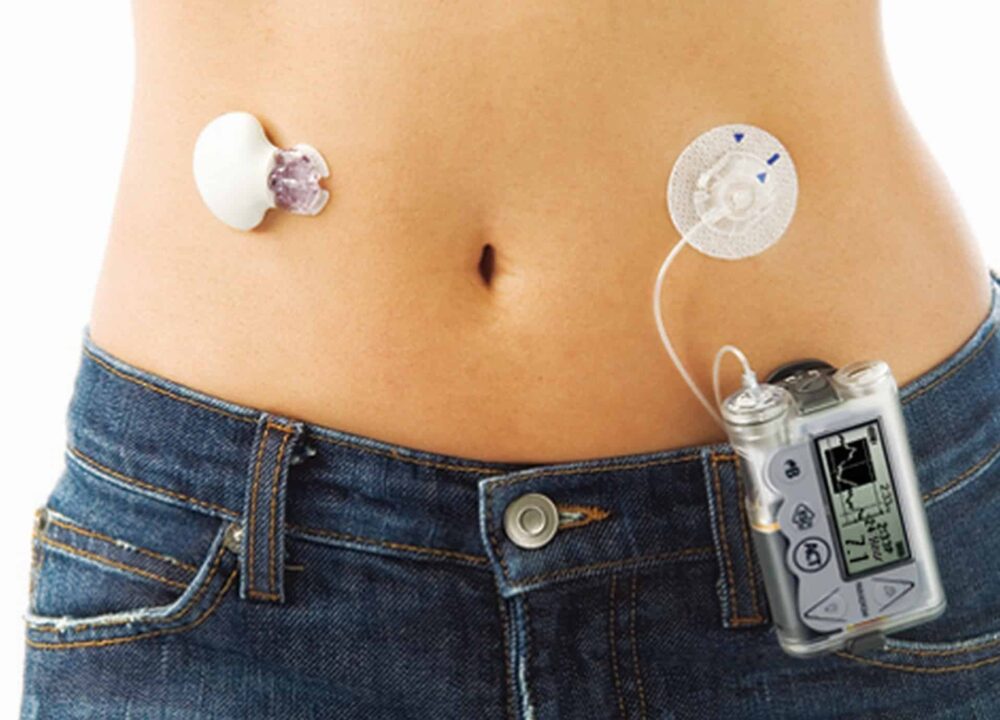
These systems use a continuous glucose monitor (CGM), an insulin pump, and a dosing algorithm (control software). The CGM streams glucose values every few minutes. The pump delivers tiny insulin adjustments at frequent intervals. The control algorithm predicts needs and modulates insulin delivery to respond to trends rather than single readings. Together, this forms an automated feedback loop.
Most platforms still require basic inputs, such as meal announcements or confirmation steps. Safety constraints limit aggressive dosing and cap insulin when glucose is falling. This layered approach helps reduce hypoglycemia risk, which remains a key safety endpoint. For broader regulatory and safety context, the U.S. FDA outlines the artificial pancreas device system pathway; this resource explains expectations for performance and safeguards.
Devices and Comparisons
System architecture varies, but common elements are shared: a CGM, an insulin pump, and a control algorithm. Differences include how meal handling works and whether users enter carbohydrates. Battery life, reservoir volume, and wearable size also matter for day-to-day comfort. In head-to-head discussions, bionic pancreas vs insulin pump comparisons often focus on automation intensity versus manual control. Users who prefer fewer decisions may value automation, while others prefer granular pump control.
Some adults still benefit from conventional basal insulin strategies, especially when insulin needs are stable. If you want a refresher on long-acting insulin options and basal dosing background, see Basaglar Cartridge for insulin pharmacology basics that inform pump settings and transitions.
Using iLet in Daily Life
Day-to-day use centers on consistent wear, sensor calibration requirements (if any), and simple prompts before meals. Practical routines—sensor changes, infusion set rotation, and site care—shape the experience as much as algorithm design. Users learn to watch trend arrows and alarms, then respond with site checks or carbohydrate intake as advised by their care team.
People frequently ask how does the ilet bionic pancreas work in practical terms. The iLet uses weight-based initialization and adaptive learning to adjust dosing, aiming to reduce carbohydrate counting demands. For therapy combinations that pair insulin with GLP-1 receptor agonists, see Xultophy Prefilled Pen for a concise overview of dual-therapy concepts. This context helps frame how adjunct treatments may interact with automated dosing.
Availability and Regulatory Status
Coverage and timelines vary by health system and insurance plan. Patients often ask when will artificial pancreas be available in their region or clinic. Availability depends on device approvals, supply chains, and local training infrastructure. U.S. regulatory resources describe the evidence required for safety, labeling, and post-market monitoring. For a general overview of regulatory expectations, the FDA’s device system guidance explains high-level principles and requirements.
Some health systems prioritize structured rollouts with phased training to ensure safe scaling. Programs usually begin with adults on intensive insulin therapy and expand as teams gain experience. If you track emerging therapies in parallel, our Mounjaro Overview provides context on drug pipelines that may complement technology advances. That perspective helps frame future combinations of devices and medications.
Costs and Insurance
Out-of-pocket costs vary by country, plan tier, and durable medical equipment coverage. Discussions of bionic pancreas cost often include sensors, transmitters, pumps, and ongoing supplies. Copays and deductibles can shift yearly, and prior authorization is common. Clinicians may supply documentation showing clinical need, prior hypoglycemia, or inadequate control on current therapy, which can support coverage requests.
Some health plans require specific medication step therapy before device approval. For medication context that frequently appears in care plans, see Trulicity Pens to understand GLP-1 options and Januvia Tablets for DPP-4 therapy background. If you prefer a broader review of treatment combinations used with or without devices, see Acceptable Combinations of Medications for comparative mechanisms and safety notes.
Benefits and Risks
Potential benefits include improved time in range, fewer lows, and reduced decision fatigue. Users also report more stable overnight control and easier responses to trend alarms. The disadvantages of artificial pancreas include device wear burden, set or sensor failures, and alarm fatigue. Users should understand backup plans for sensor downtime and infusion set occlusions. Teams often create checklists for travel, illness, and high-variability days.
Clinical organizations emphasize user education and ongoing follow-up during the first weeks. Structured training helps people interpret alerts and verify infusion site integrity. For a neutral overview of automated systems and safety mitigations, the NIDDK explains design principles and safeguards in artificial pancreas devices. For lifestyle considerations that may affect glucose control, see Alcohol and Diabetes for risk-aware guidance on drinking and hypoglycemia.
Patient Experience and Early Feedback
Early adopters often value automation during sleep and busy hours. Many also highlight fewer manual corrections and reduced mental load. Common concerns include adhesive comfort and learning how different meals affect trends despite algorithm support. Device coaching and peer support can improve comfort during the learning curve.
Reports that resemble ilet bionic pancreas reviews usually come from pilot programs, registry data, or structured interviews. These sources help teams refine education and set realistic expectations. For context on underlying physiology that shapes dosing needs, see Insulin Resistance vs. Deficiency and Diagnosing Insulin Resistance to understand insulin sensitivity changes over time.
Comparing System Approaches
Closed-loop designs differ in how they handle meals, exercise, and sensor errors. Systems that require carb announcements may deliver more precise meal boluses. Systems that avoid carb counting may rely on post-meal adjustments guided by trends. Both approaches can work when education, troubleshooting, and follow-up occur consistently.
Some users move between manual pump modes and automated modes for specific scenarios. Others prefer fully automated strategies for simplicity. These preferences often depend on lifestyle, work schedules, and comfort with alerts. For broader medication context that can complement device use, see Common Diabetes Medications for mechanism summaries and potential interactions with insulin therapy.
Research and Future Directions
Ongoing studies are testing different algorithms, sensor types, and insulin formulations. Investigators are exploring personalization features that adapt to changes in insulin sensitivity. Some groups investigate multi-hormone configurations, though most commercial systems remain insulin-only. Longer follow-up aims to measure sustained A1C changes, severe hypoglycemia rates, and device durability.
Future work on the bionic pancreas will likely focus on onboarding efficiency and equitable access. Programs may expand to broader populations once training capacity increases. As technology evolves, clinicians will balance automation benefits with the realities of wear time, supplies, and support needs. For a broad overview article that connects automation to daily life, Automating Glucose Control offers a helpful conceptual primer.
Recap
Trials in adults with type 2 diabetes show promising gains with automated insulin delivery. Systems reduce daily decision work and can improve time in range. Adoption still requires training, follow-up, and thoughtful insurance navigation. As availability expands, individual preferences and clinical history should guide device choice and setup.
Note: Always review device training materials and confirm backup plans for sensor or infusion issues.
This content is for informational purposes only and is not a substitute for professional medical advice.