Using long-acting insulin effectively starts with understanding Basaglar kwikpen and how this device works day to day. This updated guide explains the pen’s format, safe technique, dosing principles, and how it compares with other basal options.
Key Takeaways
- Pen format basics: 100 units/mL strength, multi-dose device.
- Safe use: prime, inject correctly, rotate sites, dispose properly.
- Dosing: follow your prescriber; conversions require clinical oversight.
- Costs vary by coverage; explore savings programs and assistance.
Basaglar kwikpen: What It Is and How It Works
This prefilled pen delivers insulin glargine, a long-acting basal insulin (background insulin). It provides a relatively flat activity profile that can help stabilize fasting and between-meal glucose. Many adults inject once daily at the same time, although clinical plans may differ by person and regimen.
Insulin glargine is a long-acting formulation designed to precipitate in subcutaneous tissue, releasing slowly over many hours. That pharmacology supports baseline insulin needs while mealtime insulin covers food. For broader context on non-identical follow-on and biosimilar products, see Biosimilar Insulin to understand category differences and regulatory terms.
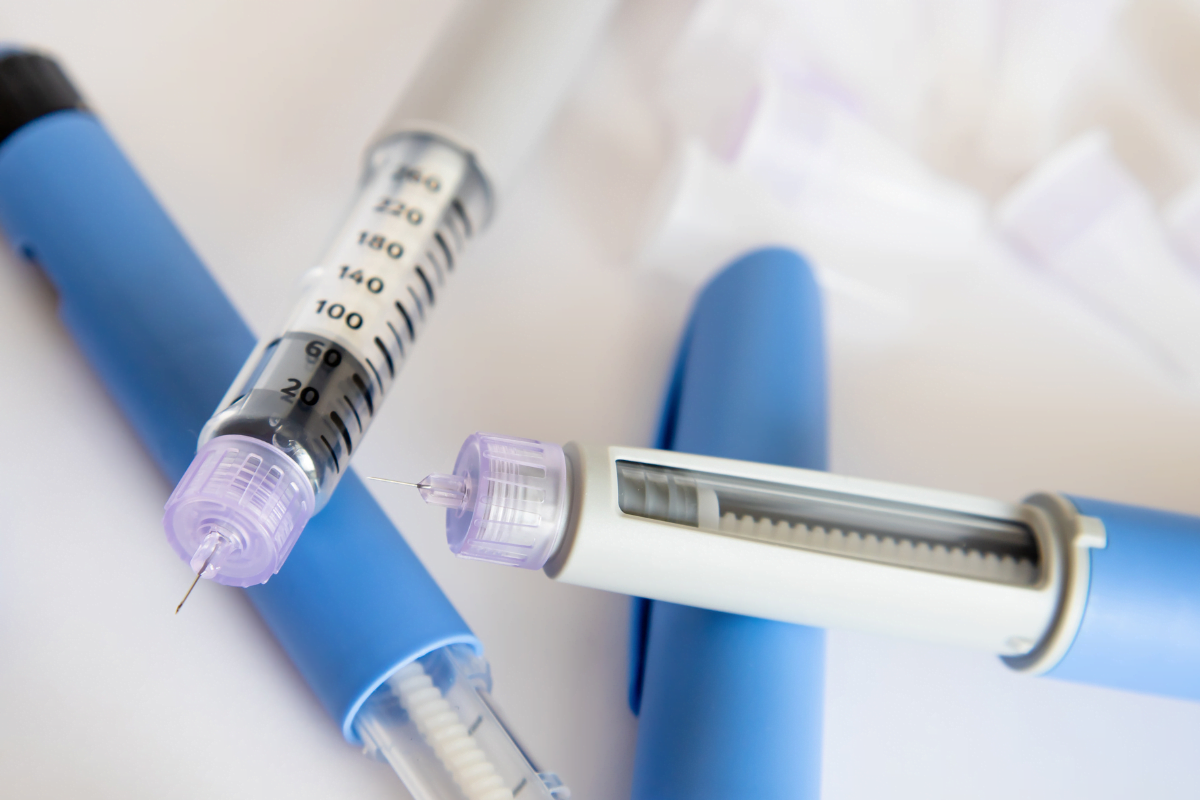
Pen Format, Strength, and Needles
This pen contains a 100 units/mL concentration in a multi-dose, prefilled device. Each pen typically holds 3 mL of solution, equal to 300 total units, though packaging can vary by market. Most boxes include several pens, commonly five, helping support a month or more of basal therapy depending on your prescribed dose. Always check the carton for the exact count and local labeling specifics.
If you wonder how many units in basaglar kwikpen, the usual total is 300 units per pen at 100 units/mL. Needle compatibility is broad; standard pen needles in the 4–8 mm range are commonly used. Most packages do not include needles, so confirm availability before starting. For detailed storage and device considerations, you can review the Basaglar Cartridge Guide for a complementary overview of formulation and handling.
Authoritative product specifications, such as concentration and device instructions, are detailed in the manufacturer’s Basaglar Instructions for Use, which outline pen parts, priming, and injection steps.
How to Use the Pen Safely and Correctly
Good technique improves dose accuracy and stability. Wash hands, attach a new needle, and prime the pen to clear air. Dial the prescribed dose, choose an approved site (abdomen, thigh, or upper arm), and inject at the correct angle. Hold the needle in place for the recommended number of seconds to ensure full delivery. Rotate injection sites to reduce skin complications and avoid repeated use of the same area.
If you need a step-by-step refresher on basaglar kwikpen how to use, a structured checklist helps. Confirm you have the right insulin and strength, inspect the solution, and follow priming volume per labeling. Learn the signs of delivery problems, like unusual resistance. For a detailed walkthrough with diagrams, see Basaglar Pen Instructions for visual steps and priming details. Additional procedural details are also listed in the FDA-cleared FDA label to verify device-specific recommendations.
Dosing Basics and Conversions With Other Basal Insulins
Dose decisions should remain individualized and guided by your prescriber. Basal insulin dosing considers A1C goals, fasting glucose, hypoglycemia risk, and concomitant medications. Titration plans often adjust by small increments and at measured intervals. Patients switching between basal products may require more frequent glucose checks during transitions to guard against hypo- or hyperglycemia.
Regarding basaglar and lantus dose conversion, these glargine U‑100 products are generally approached with similar dosing principles, but prescribers confirm clinical equivalence and monitor closely. Differences in devices, injection timing, and patient factors still matter. For device comparison context, see Lantus SoloStar Pens 100 Units/ml when considering format, and review the Basaglar–Lantus Comparison for a clinical overview. Broader dosing frameworks appear in the ADA Standards of Care, which outline basal insulin use principles.
Side Effects and Safety Checks
Common basaglar side effects may include hypoglycemia (low blood sugar), injection site reactions, and mild edema. Less common events include allergic reactions and changes in fat tissue at injection sites (lipodystrophy). Risk can increase with missed meals, extra activity, alcohol, or drug interactions that potentiate insulin action. Carry glucose sources and monitor levels more often when routines change.
Serious but uncommon concerns include severe hypoglycemia and hypersensitivity. Inform clinicians about all medications, especially agents affecting glucose or potassium balance. Rotate injection sites and inspect skin routinely to reduce local issues. For detailed safety information, dosing instructions, and contraindications, consult the official FDA label, and review our practical overview in Side Effects Best Practices for self-check strategies.
Costs, Coverage, and Ways to Save
Out-of-pocket costs depend on pharmacy contracts, insurance tiers, deductibles, and discounts. Manufacturer assistance and nonprofit programs may help qualified patients reduce expenses. State or provincial policies can also influence co-pays and access. Medicare and commercial plans frequently maintain formularies that list preferred basal insulins, sometimes requiring prior authorization for alternatives.
For those facing high basaglar cost without insurance, explore negotiated pharmacy pricing, patient assistance programs, and savings cards. To understand cost drivers such as supply chain, demand, and coverage policies, see Basaglar Insulin Price Factors for a deeper breakdown. For practical savings strategies, visit Basaglar Coupon Tips for program types and enrollment notes. You can also review Type 2 Diabetes resources for broader management context.
Storage, Handling, and Disposal
Proper storage protects dose potency and consistency. Unopened pens are typically refrigerated until first use, then stored at room temperature for a limited in-use period specified on the label. Avoid freezing or excessive heat, and discard pens that were frozen, overheated, or past in-use dating. Keep pens capped between injections to reduce contamination risk and insulin degradation from light exposure.
Used needles require a sharps container or equivalent rigid disposal system per local regulations. Follow household hazardous waste guidance or pharmacy take-back programs where available. If you need a reference while traveling, keep a copy of the device’s official storage instructions. You can verify time and temperature limits in the manufacturer’s Basaglar Instructions for Use. For a refresher on safe handling of a basaglar insulin pen, see the packaging insert and consider reviewing our Basaglar Cartridge Guide for storage rationales and practical examples.
Comparing Long-Acting Options
Insulin glargine U‑100 appears in multiple brands, while other basal insulins use different molecules or concentrations. Clinical performance can vary by duration, peak profile, and dose flexibility. When discussing basaglar vs lantus, both contain insulin glargine U‑100, with device and support program differences. Toujeo (insulin glargine U‑300) and Tresiba (insulin degludec U‑100/U‑200) are higher-duration options that may alter dosing needs under clinician guidance.
To compare design and duration details, explore Toujeo DoubleStar Prefilled Pen for concentrated glargine device notes, and Tresiba FlexTouch Pens for different duration profiles. For head-to-head editorial context, see Basaglar vs Levemir to contrast detemir-based basal insulin. You can also review What Is Tresiba for pharmacology and use cases beyond glargine.
Recap
Successful basal insulin use blends sound device technique, individualized dosing, and steady monitoring. Confirm the insulin, prime correctly, inject safely, and rotate sites. Coordinate with your care team when considering switches, because pharmacology and coverage can differ. Use authoritative instructions and reliable comparisons to maintain safety and consistency.
Note: Keep glucose treatment options available during daily activities, and review sick-day plans with your clinician ahead of time.
This content is for informational purposes only and is not a substitute for professional medical advice.