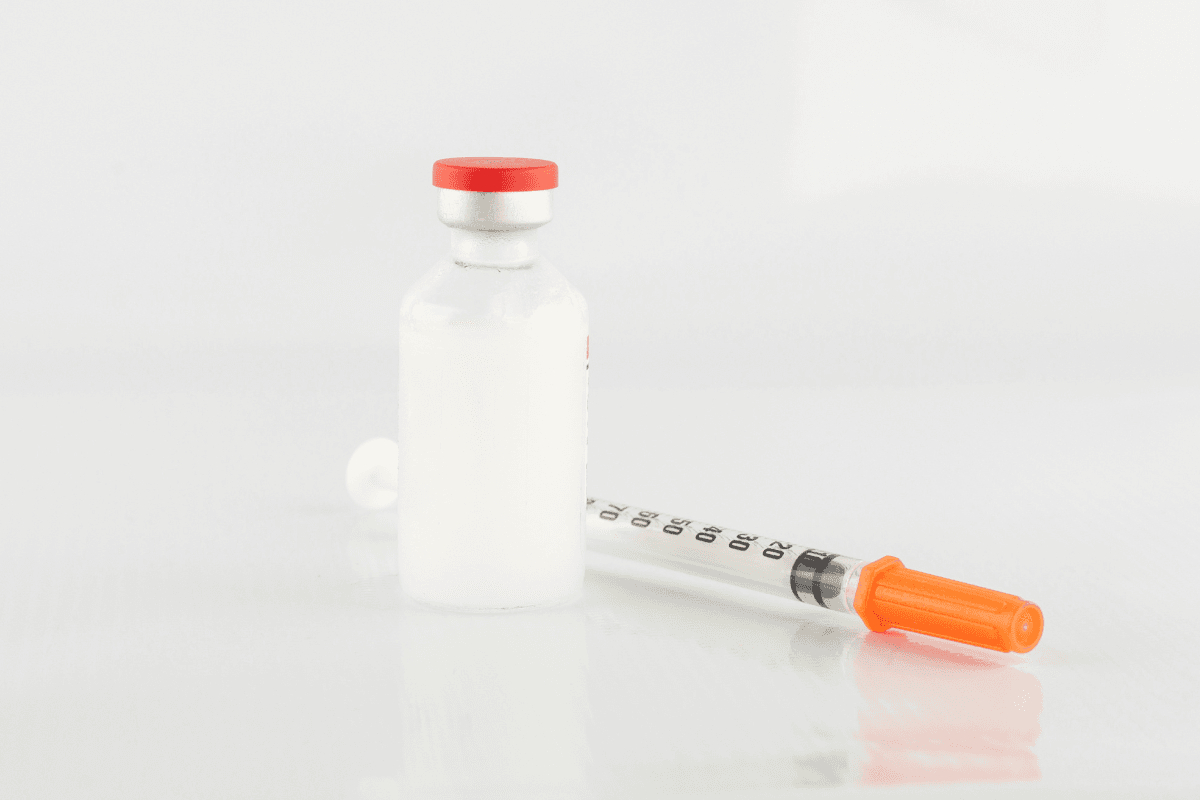
Knowing how to dispose of expired insulin protects people, pets, and waste workers. This guide explains how to dispose of expired insulin, what belongs in a sharps container, and what does not. You will also learn storage steps that reduce waste, plus community options when home disposal is not appropriate.
Key Takeaways
- Sharps safety first: seal and label puncture-resistant containers.
- Do not flush insulin; follow local medicine take-back rules.
- Separate needles from vials and pens before disposal.
- Store correctly to prevent waste and potency loss.
- Use pharmacy or municipal programs when available.
How to Dispose of Expired Insulin
Start by separating components. Needles, pen needles, and lancets are sharps. Vials, cartridges, and pens without needles are not sharps. Keep sharps in a dedicated, puncture-resistant container with a tight lid. A red FDA-cleared sharps container is preferred. If you do not have one, use a heavy-duty plastic bottle with a screw cap, then label it clearly as “Do Not Recycle – Household Sharps.”
For the insulin liquid inside vials or cartridges, avoid sinks and toilets. When a medicine take-back program is unavailable, many jurisdictions allow mixing the residual liquid with an unappealing material (e.g., used coffee grounds or cat litter), sealing in a bag, and placing it in household trash. Always remove or black out personal information on labels. Verify local rules before discarding; city or provincial regulations may differ.
For practical device handling background, compare delivery options in Insulin Pen vs Syringe for context on components you may need to discard.
Sharps: Syringes, Pen Needles, and Lancets
Used needles, pen needles, and lancets can puncture bags and harm sanitation staff. Place each immediately into a sharps container; never recap, bend, or break needles. When the container is about three-quarters full, seal it permanently. Many pharmacies, clinics, and local waste services offer sharps drop-off or mail-back programs. Follow any regional labeling requirements, such as the biohazard symbol or contact information.
Do not place sharps in curbside recycling. This contaminates sorting lines and puts workers at risk. The U.S. Food and Drug Administration provides detailed guidance for home users on safe sharps disposal, which aligns with common municipal programs. Needles and lancets are never part of the fda flush list, so do not flush or pour them.
For device-specific needle details and sizes, see Insulin Pen Needles for usage and safety tips, or review BD Needles for brand-specific features that may influence safe handling.
Vials and Cartridges: Liquid Contents and Non-Sharp Parts
Vials, cartridges, and pen bodies without attached needles are not sharps, but they still need proper handling. If a pharmacy or municipal medicine take-back is available, use that route first for the liquid and the original containers. When no take-back is accessible, follow local guidance for household disposal. Many areas permit solidifying small volumes before trashing.
To limit diversion and environmental exposure, mix the contents with an unpalatable absorbent, seal in a bag, hide it within household trash, and remove personal data from labels. The FDA describes safe household steps for medicine disposal at home, which you can adapt to insulin liquids. These instructions cover how to dispose of liquid meds when take-back programs are not available in your area.
For protecting vials between uses, see Insulin Vials Accessories for breakage prevention ideas that also reduce disposal volume from accidents.
Storage to Minimize Waste
Good storage prevents needless discards. Follow the package insert and your clinic’s guidance. Keep unopened insulin in the refrigerator, and avoid freezing or excessive heat. After first puncture or activation, most products have a room-temperature “in-use” window. Track the date you opened each vial or pen. Use reminders on the box or your phone to prevent accidental overuse beyond labeled timelines.
Review manufacturer and clinical recommendations on insulin storage practices from a national diabetes organization. For a broader overview of product stability and signs of problems, the Expired Insulin Guide explains potency changes, visual checks, and handling after the in-use period. If you use cartridges, see Insulin Cartridges for handling steps that lower waste from device mishaps. To manage your daily device routine, this primer on Use an Insulin Pen can help avoid premature discards.
Some labels describe insulin storage after opening in days, while others specify hours or temperatures. Always check your specific product’s instructions, including any limits on light exposure or agitation.
Temperature Excursions and Stability
Accidental warmth or cold can reduce potency. Document what happened, how long, and the approximate temperature. When in doubt, consult the product insert or your provider before continued use. Real-world scenarios happen, such as insulin left out of fridge for 2 hours during a commute or at work. Brief exposures within labeled in-use conditions may be acceptable, but do not refreeze or heat insulin to compensate.
Watch for clumping, threads, frosting, or unexpected glucose trends after a temperature incident. If readings drift without another cause, replace the product and dispose of it safely. For pet owners, Pet Insulin Storage highlights temperature and handling nuances that can prevent waste and extra discards in veterinary use.
Community Programs and Institutional SOPs
Local pharmacies often accept medicines or sharps through take-back bins or scheduled events. Ask whether they accept insulin liquids and what preparation is required. Some programs require the original container with labels; others ask for sealed bags. If no local bin exists, your municipality may offer mail-back options or a hazardous waste day. Always confirm rules before traveling with full sharps containers or liquid medicines.
Hospitals and clinics follow facility procedures for biomedical waste, which the public should not use. Instead, ask your pharmacist how to dispose expired medicine in pharmacy programs serving your area. For broader reading across therapy topics and disposal-adjacent issues, explore our Diabetes Articles hub. Device users may also benefit from reviewing NovoPen 4 features because correct dosing mechanics reduce avoidable wastage.
Why Safe Disposal Matters
Improper discards can injure sanitation workers and contaminate sorting lines. Needlestick injuries are preventable with proper containers and drop-off programs. Pouring liquids down drains risks environmental contamination and violates local rules. Follow evidence-based steps to protect your community and household. The FDA’s consumer guidance on unused medicine disposal outlines practical, legal, and safety considerations.
Using degraded insulin can undermine glucose management. The phrase what happens if you use expired insulin reflects real-world risks: unexpected hyperglycemia, variability, and unplanned clinical visits. If you suspect degradation, replace the product, then discard it using the steps above. Monitor levels closely using your meter; consistent readings help confirm stability. For testing supplies and trend tracking, see Contour Test Strips or OneTouch Verio Test Strips as examples of tools used to assess control.
Recap
Separate sharps from non-sharps, contain needles safely, and never flush insulin. Use take-back programs when available; otherwise, follow local rules for solidifying liquids and sealing containers. Store correctly to avoid waste and safety risks. When storage errors happen, replace suspect products and discard them responsibly.
Tip: Keep a small, labeled sharps container in your travel kit to avoid unsafe improvisation away from home.
Note: Local regulations vary. When unsure, ask your pharmacist or public health office for disposal guidance.
This content is for informational purposes only and is not a substitute for professional medical advice.