Iron overload disorders can damage the liver, heart, and pancreas if they go unnoticed. Hemochromatosis is the most common genetic cause, yet it is often underdiagnosed. This guide explains core concepts in plain language and clinical terms, so you can interpret tests, understand risks, and discuss care options confidently.
Key Takeaways
- Early recognition helps prevent liver, heart, and endocrine complications.
- Transferrin saturation and ferritin guide screening and monitoring.
- Genetic testing confirms most hereditary cases linked to HFE variants.
- Therapeutic phlebotomy remains the first-line treatment for iron removal.
- Family screening and periodic labs reduce long-term disease burden.
What Is Hemochromatosis?
Clinically, hemochromatosis refers to conditions that cause excess intestinal iron absorption and tissue iron deposition. In plain terms, the body stores too much iron over time, slowly harming organs. Hereditary types involve pathogenic variants in iron-regulation genes, most commonly HFE (High Iron, Fe), while secondary forms arise from other diseases or repeated transfusions.
The condition was historically nicknamed “bronze diabetes” due to skin hyperpigmentation and glucose dysregulation. Because metabolic and endocrine disorders often intersect, see the Endocrine Thyroid overview for broader context on related conditions for additional background. For a wider endocrine view, see Endocrine Thyroid for topic grouping and related articles.
For foundational clinical background and patient guidance, the National Institute of Diabetes and Digestive and Kidney Diseases provides a concise overview of diagnosis and treatment; review the NIDDK hemochromatosis page for current definitions and care principles.
Iron Overload: Types and Inheritance
Hereditary forms include HFE-related disease (classical adult-onset), juvenile-onset disease (HJV or HAMP variants), transferrin receptor 2 (TFR2)–related disease, and ferroportin (SLC40A1) disease. Most HFE-related cases follow autosomal recessive patterns, while ferroportin disease is typically autosomal dominant. Expressivity varies, influenced by age, sex, alcohol use, viral hepatitis, and metabolic comorbidities.
Family history helps prioritize screening, especially among first-degree relatives. When discussing hemochromatosis inheritance, clinicians explain that two pathogenic HFE alleles usually confer greatest risk for iron overload, but not everyone with these variants becomes symptomatic. Carriers generally have normal iron levels, though mild abnormalities may appear under specific conditions like inflammation, pregnancy, or significant alcohol use.
For gene basics, MedlinePlus Genetics outlines how HFE regulates iron uptake and where it sits in the genome. See the HFE gene summary for variant types and chromosomal location.
Causes and Risk Factors
Primary disease stems from dysregulated iron absorption in the duodenum, driven by gene variants that reduce hepcidin’s regulatory control. When exploring hemochromatosis causes, think beyond genes. Contributing risks include heavy alcohol use, chronic hepatitis, nonalcoholic fatty liver disease, and iron-rich supplementation without indication.
Additional factors can amplify iron accumulation over time. Men often manifest earlier due to lack of physiologic iron losses. Women may present later, as menstruation and pregnancy can offset iron burden until middle age. Coexisting metabolic syndrome and obesity can exacerbate hepatic injury in individuals with iron overload.
Secondary Iron Overload
Not all iron overload is hereditary. Transfusion-dependent anemias, chronic hemolysis, ineffective erythropoiesis, and some chronic liver diseases can drive secondary accumulation. Clinicians evaluate overall transfusion history, marrow disease, and inflammatory markers when phenotypes do not fit classical patterns.
When assessing secondary hemochromatosis causes, consider repeated transfusions, thalassemia syndromes, myelodysplastic syndromes, and advanced chronic liver disease. Management in these scenarios often differs from hereditary forms, and chelation may be preferred when phlebotomy is impractical due to anemia or comorbidities.
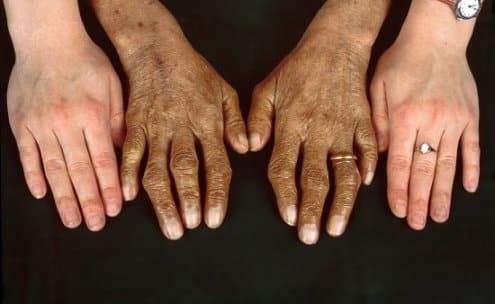
Signs and Symptoms Across Sex and Age
Early symptoms may be subtle and nonspecific: fatigue, reduced exercise tolerance, arthralgia, or abdominal discomfort. Over time, patients can develop skin bronzing, arthropathy of the second and third metacarpophalangeal joints, hypogonadism in men, or amenorrhea in women. Cardiac involvement can lead to arrhythmias or cardiomyopathy, while pancreatic damage may cause impaired glucose tolerance or diabetes.
Women may have delayed onset of clinical features because of menstrual iron losses, making presentations later and sometimes underrecognized. Foot pain and stiffness can reflect iron-related arthropathy, especially in weight-bearing joints. Because overlap with diabetes is common, readers interested in the spectrum of diabetes phenotypes can review Type 3 vs 3c Diabetes for a comparison that clarifies nomenclature and organ involvement.
Diagnosis and Criteria
Clinicians start with iron indices. Elevated fasting transferrin saturation (often ≥45%) plus raised ferritin suggests iron overload. To establish hemochromatosis diagnosis, most guidelines recommend HFE genetic testing when iron studies are abnormal and secondary causes are excluded. Persistently high ferritin with normal saturation prompts evaluation for inflammation, fatty liver, or malignancy rather than primary iron loading.
Noninvasive liver iron quantification by MRI can stage hepatic burden without biopsy. Liver biopsy remains reserved for specific scenarios, such as evaluating advanced fibrosis when noninvasive tests are indeterminate. For current expert criteria and care pathways, see the GeneReviews guidance summarizing evaluation and management of hereditary forms.
Interpreting Lab Results
A standard hemochromatosis labs panel includes fasting serum iron, transferrin saturation, and ferritin, often with ALT/AST to screen for hepatic injury. Transferrin saturation reflects circulating iron availability, while ferritin signals stored iron and responds to inflammation. In iron overload, TIBC typically trends low-to-normal, and saturation rises, whereas purely inflammatory states can elevate ferritin without high saturation.
MedlinePlus explains how iron tests complement one another across conditions; see the iron tests overview for what each assay measures. When diabetes coexists, insulin therapy may be part of comprehensive care; for safe injection technique and device choices, the Insulin Pen Needles guide outlines options and practical tips readers can apply.
Treatment and Long-Term Management
First-line hemochromatosis treatment typically involves scheduled therapeutic phlebotomy to remove iron and lower ferritin toward a maintenance target. In secondary iron overload with anemia, iron chelation may be considered instead. Clinicians advise avoiding unnecessary iron supplements, limiting alcohol, and monitoring for viral hepatitis and metabolic liver disease.
Weight management and cardiovascular risk reduction remain important because cardiometabolic stress can worsen hepatic outcomes. For context on metabolic therapies and outcomes, see GLP-1 Weight Loss Drugs discussing how GLP-1 agents influence weight and cardiometabolic risk. If diabetes requires insulin, readers can review Premixed Insulin Guide for regimen structure, and Insulin And Weight Gain for strategies that may help limit weight changes during therapy.
Therapeutic Phlebotomy and Guidance
Protocols aim to deplete excess iron during induction, then maintain safe levels. Typical induction removes 400–500 mL per session at intervals based on ferritin and hemoglobin. During maintenance, intervals lengthen to keep ferritin in the low-normal range without causing anemia. Monitoring hemoglobin before each draw helps prevent symptomatic drops.
When discussing hemochromatosis treatment phlebotomy, clinicians individualize targets using comorbidity, age, and liver status. Authoritative reviews outline reasonable ferritin goals and monitoring cadence; consult the NIDDK overview for general principles and patient-facing details. Note: Chelation therapy is usually reserved for secondary forms with anemia or when phlebotomy is not feasible, and should be supervised by specialists.
Complications and Outlook
Untreated iron overload increases risk of cirrhosis, hepatocellular carcinoma, cardiomyopathy, and endocrine dysfunctions, including diabetes and hypogonadism. Early detection and iron reduction improve outcomes substantially. After iron depletion, many patients stabilize, and some symptoms such as fatigue may improve, though established arthropathy often persists.
Cardiometabolic health matters for long-term risk. For a look at cardioprotective outcomes with incretin-based therapies in diabetes management, see Mounjaro Heart Benefits summarizing heart-related endpoints in clinical research. People using insulin should also understand delivery devices; the Insulin Cartridges Guide can help when clinicians adjust regimens for coexisting diabetes.
Living Well and Follow-Up
Ongoing care includes periodic ferritin and transferrin saturation, liver assessment when indicated, and evaluation for joint, endocrine, and cardiac involvement. Family members may benefit from targeted testing after a proband is identified, following local guidelines and shared decision-making. Clinicians generally advise moderation with alcohol, mindful vitamin C intake with iron-rich meals, and avoiding unnecessary iron supplements.
For those at risk of hypoglycemia due to glucose-lowering therapies, preparedness matters. The Glucagon Injection Kit page provides product details that patients may discuss with their care team as part of safety planning. Tip: Keep a personal lab trend sheet; patterns over time often reveal issues before symptoms emerge.
Recap
Iron overload disorders are manageable when identified early. Clear testing, careful interpretation, and appropriate iron reduction can limit organ damage and improve quality of life. Coordinate with specialists for genetic counseling, liver assessment, and tailored maintenance plans as needs evolve.
This content is for informational purposes only and is not a substitute for professional medical advice.