Toujeo insulin is a long-acting form of insulin glargine designed for steady, once-daily basal coverage. It releases slowly after subcutaneous (under the skin) injection, which helps stabilize fasting and between-meal glucose. Understanding its action, safe use, and limitations supports safer day-to-day diabetes care.
Key Takeaways
- Steady basal coverage: designed for consistent, 24-hour background insulin.
- Pen-based delivery: dose in small increments for flexible titration.
- Safety first: watch for low blood sugar and GI symptoms.
- Store correctly: keep unopened pens refrigerated; protect from heat.
- Compare thoughtfully: consider alternatives if needs or patterns change.
Toujeo insulin: What It Is and How It Works
Toujeo is insulin glargine U-300, a concentrated basal insulin analog. After injection, microprecipitates form in the subcutaneous tissue and slowly release insulin over many hours. This extended absorption helps reduce peaks and may lower day-to-day variability. Steady delivery can simplify background coverage for adults with type 1 or type 2 diabetes.
Mechanistically, insulin glargine binds to insulin receptors and facilitates cellular glucose uptake while suppressing hepatic glucose output. The formulation’s higher concentration allows a smaller injection volume for the same number of units. For labeled pharmacology, see the prescribing information (target=”_blank” rel=”noopener”) prescribing information. For historical context on basal designs and evolution, see Evolution of Insulin Therapy for long-acting design background and modern options overview.
Using the Pen Devices
Consistent technique improves accuracy and comfort. Wash hands, attach a new needle, prime per device instructions, dial the dose, and inject into lifted skin at a recommended site. Hold the needle in place for the full count before withdrawing. Rotate sites within the same region to reduce lipohypertrophy (fatty tissue thickening) and improve absorption.
If you are new to pen injections, ask your care team for hands-on training or a step-by-step guide. Start with simple routines and a reminder system for daily timing. For dosing principles and adjustment context, see Toujeo Dosage Guide for starting frameworks and titration concepts. Many patients also watch short device tutorials to reinforce safe handling.
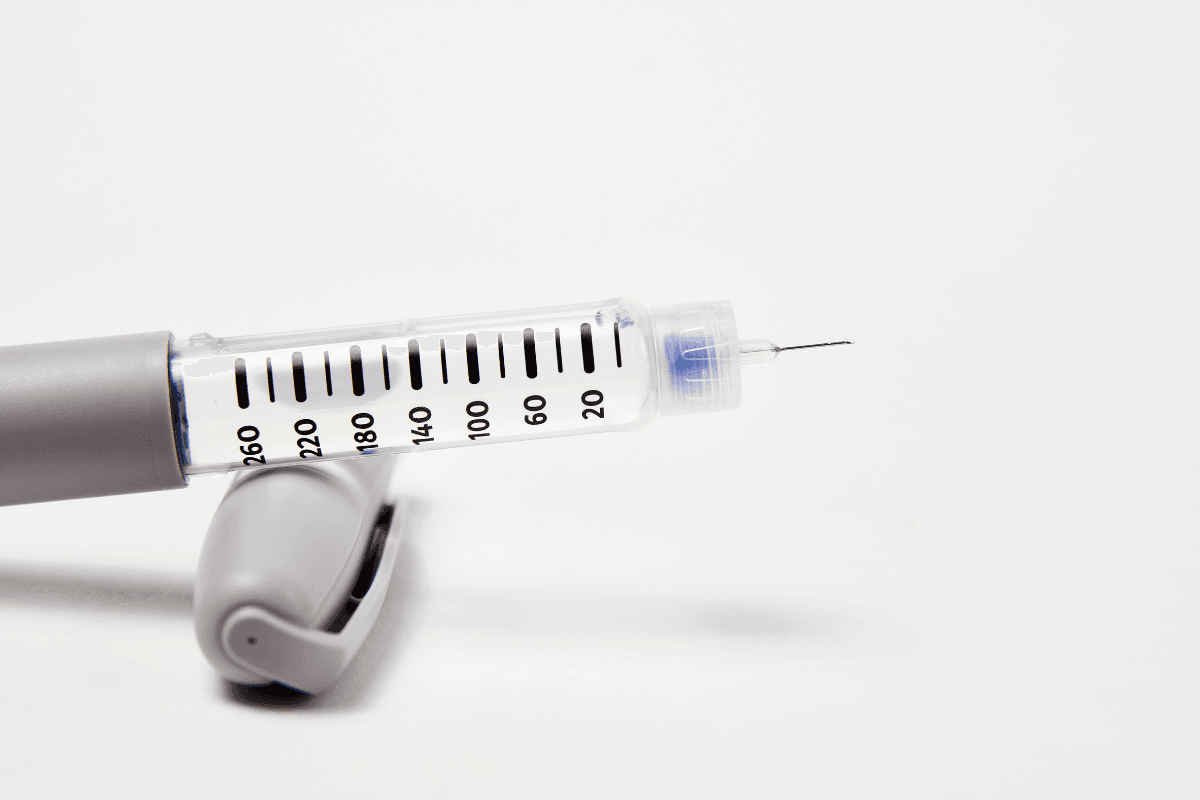
SoloStar Device Details
The toujeo solostar pen is a prefilled device calibrated for the U-300 formulation. Dosing increments are designed to help fine-tune basal needs with fewer clicks for higher unit requirements. Always confirm the selected dose in units, not milliliters, before injection. Dispose of used needles in a puncture-resistant sharps container.
Some patients require higher total daily doses and benefit from a larger-capacity device. For product specifications and capacity details, see the Toujeo DoubleStar Prefilled Pen for high-capacity U-300 pen specifications. If your regimen includes mealtime insulin, note that pens are not interchangeable between brands or concentrations.
Safety and Side Effects
Common toujeo insulin side effects include hypoglycemia symptoms such as shakiness, sweating, and confusion. Gastrointestinal effects like nausea can occur; diarrhea is reported less often. Rotate sites to reduce local injection reactions, and monitor patterns with a glucose meter or CGM. Carry fast-acting carbohydrates and review low-glucose protocols with your clinician.
Insulin needs change with weight, kidney function, illness, and activity. Medications like steroids can raise glucose and increase insulin requirements. For consensus safety guidance, see the American Diabetes Association Standards of Care for insulin therapy principles. For symptom patterns and practical tips, visit Toujeo Side Effects for management strategies and escalation thresholds.
Weight Gain and Metabolism
Many people ask why does toujeo cause weight gain. Several mechanisms contribute: improved glycosuria reduces calorie loss in urine, insulin promotes anabolism (tissue building), and defensive eating after lows adds excess calories. Fluid shifts may also occur early with better glycemic control. The effect varies by dose, diet pattern, and activity level.
To limit weight changes, emphasize consistent meals, fiber, and protein while spacing carbohydrates across the day. Review low-glucose prevention strategies to reduce corrective snacking. Strength training may improve insulin sensitivity and body composition. For comparison within the same analog class, see Lantus Side Effects to understand glargine class effects and how they overlap.
Storage and Stability
Proper storage protects potency and consistency. Unopened pens are typically refrigerated until first use; in-use pens are usually kept at room temperature, away from heat and light. Do not freeze, and avoid leaving pens in vehicles or direct sunlight. If insulin looks cloudy or has particles, do not use it.
Check the device’s in-use time window and discard after it expires, even if insulin remains. Each manufacturer sets temperature limits and timelines. For official thresholds and time limits, see the prescribing information and the FDA U-300 glargine label for storage instructions and stability data. If unsure about handling, ask your pharmacist to review practical steps.
Comparing Basal Options
Patients often weigh toujeo vs lantus when choosing a basal insulin. Both contain insulin glargine but at different concentrations and delivery profiles. Newer long-acting options and biosimilar products may also fit certain needs or coverage requirements. Discuss with your clinician how fasting patterns, lifestyle, and device preferences influence the decision.
For broader context on alternatives, see Tresiba vs Lantus for differences among long-acting analogs. If considering glargine biosimilars, review the Basaglar Cartridge for product characteristics and delivery format, and see Biosimilar Insulin for regulatory definitions and interchangeability concepts.
Long-Term Use and Monitoring
Long-term basal insulin use centers on stable glucose, minimized hypoglycemia, and reduced variability. Periodic A1C, time-in-range metrics, and nocturnal patterns help confirm appropriateness. Weight trends, kidney function, and hypoglycemia awareness should be reviewed regularly. Adjustments may be needed with aging, new comorbidities, or medication changes.
Coordinate education on sick-day rules and travel plans, including extra supplies and temperature protections. If your team recommends combination therapy, fixed-ratio options that include basal insulin plus an incretin-based agent may simplify administration. For background on such combinations, see Soliqua SoloStar Pens for an overview of the glargine/lixisenatide pairing.
Dose Boundaries and Overdose Awareness
Insulin dosing is individualized. Signals of possible overdose include prolonged or recurrent hypoglycemia, confusion, visual changes, and, in severe cases, seizures. Alcohol intake, missed meals, or unexpected exertion can increase risk. Keep a source of rapid carbohydrates available and know when to escalate for urgent care.
Never re-dose for a missed injection without professional guidance, and do not attempt unit conversions across different concentrations. If you are evaluating dosing adjustments, consult your prescriber first. For structured frameworks, see the Toujeo Dosage Guide for dosing ranges and titration context to discuss at your next appointment.
Tip: Set a consistent daily alarm and use a written log or app to track doses, injection sites, and any symptoms worth reviewing later.
This content is for informational purposes only and is not a substitute for professional medical advice.